Back to Journals » Veterinary Medicine: Research and Reports » Volume 6
Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes
Authors Heatley JJ , Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G
Received 19 July 2014
Accepted for publication 16 September 2014
Published 16 January 2015 Volume 2015:6 Pages 61—69
DOI https://doi.org/10.2147/VMRR.S71402
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Takashi Agui
J Jill Heatley,1 Jennifer Cary,2,3 Lyndsey Kingsley,1 Hughes Beaufrere,4 Karen E Russell,5 Gary Voelker2,3
1Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, 2Department of Wildlife and Fisheries Sciences, 3Texas A&M Biodiversity Research and Teaching Collections, Texas A&M University, College Station, TX, USA; 4Health Sciences Centre, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada; 5Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, College Station, TX, USA
Abstract: Feasibility and effect of midazolam administration on blood analytes and for sedation of Passeriformes being collected in a larger study of genetic biodiversity was assessed. Midazolam (5.6±2.7 mg/kg) was administered intranasally prior to sampling, euthanasia, and specimen preparation of 104 passerine birds. Each bird was assessed for sedation score and then multiple analytes were determined from jugular blood samples using the i-STAT® point of care analyzer at “bird side”. Most birds were acceptably sedated, sedation became more pronounced as midazolam dose increased, and only a single bird died. Electrolyte concentrations and venous blood gas analytes were affected by midazolam administration while blood pH, packed cell volume, hemoglobin, and calculated hematocrit were not. Intranasal midazolam gives adequate sedation and is safe for short-term use in free-living Passeriformes. Based on venous blood analyte data, sedation of Passeriformes prior to handling appears to reduce stress but also produces venous blood gas differences consistent with hypoventilation relative to birds which were not given midazolam. Further study is recommended to investigate midazolam's continued use in free-living avian species. Studies should include safety, reversal and recovery, effect upon additional endogenous analytes, and compatibility with studies of ecology and toxicology associated with pollution or other environmental degradation in Passeriformes.
Keywords: Avian, benzodiazepine, biochemistry, blood gas, electrolyte, clinical pathology
Introduction
Sampling for evaluation of hematological and biochemical analytes is a useful, nonlethal, and minimally invasive method to determine health status of the individual bird and avian populations.1–8 However, venipuncture and blood sample collection from Passeriformes is often hindered by patient size and temperament as well as clinical skill of the sample collector. Furthermore, the effect of capture and handling stress on various blood analytes is difficult to control and account for in field studies.9 Midazolam is a short-acting benzodiazepine with potent anxiolytic, amnestic, hypnotic, anticonvulsant, muscle relaxant, and sedative properties, convenient for use in animals based on a short elimination half-life, water solubility allowing multiple administration options, rapid onset of action, reversibility, and minimal cardiovascular effects.10–12 Midazolam has been administered intranasally to sedate parrots, canaries Serinus canarius, finches Taeniopygia guttata, and other avian species for research and clinical case management.11–16 Sedation with midazolam in birds decreases vocalization, flight, and defense responses, and facilitates handling for diagnostic and minor therapeutic procedures.11,12,17,18 Additional study of midazolam has been recommended for “reducing stress in captured wild birds”.14
The objective of this prospective cross-sectional study was to assess feasibility and safety of the sedative midazolam administered intranasally to Passeriformes in a field setting. Midazolam was hypothesized to be an easily administered, rapid-onset, effective, and safe sedative for Passeriformes to facilitate handling and sample collection. Midazolam’s effect on select venous blood analytes was also assessed by comparing those obtained in this study to a study that assessed Passeriforme health in nonsedated birds.19 Some nonspecific measures of stress such as lactate or glucose concentrations were also hypothesized to differ based on administration of midazolam. These findings are the first large-scale assessment of midazolam sedation in free-living Passeriformes and the first report of the effect of midazolam on venous blood analytes in these species.
Materials and methods
Specimen collection
In conjunction with an ongoing specimen collection for assessment of avian genetic variation and biodiversity in Texas, 104 Passeriformes were sampled from March to June 2012 from eight counties of Texas (Bastrop, Caldwell, Comal, Grimes, Hays, Kerr, Travis, Williamson). Coordinates of sampling spanned 29.47.686–30.55.011 N latitude and 96.05.604–99.23.731 W longitude. Time of day for sampling ranged from 7.30 am to 12.45 pm central standard time. Season, region, time of day, and method of capture of birds were similar to a previous study in which sedation was not administered.19 Research was conducted under required Texas Parks and Wildlife and United States Fish and Wildlife Permits and with approval of the Texas A&M University Institutional Animal Care and Use Committee.
Animal sampling
Birds were captured via mist net and placed in cloth bags until administration of midazolam (5 mg/mL injection USP; Hospira, Inc., Lake Forest, IL, USA) intranasally at a dose of 5.55±2.66 (mean ± standard deviation [SD], range 2.39–13.5) mg/kg.12,15,17 Birds were initially dosed based on weights (Table 1) generalized from known species weight ranges; exact dosages were later recalculated using postmortem frozen bird weight. Birds were then held in bags for at least 5 minutes to allow calming and sedation to occur before sampling. Sedation score was determined based on a modified sedation scale (Table 2).20,21 Animals were manually restrained for collection of 0.2–0.5 mL of blood via jugular venipuncture with needle and syringe. Jugular venipuncture was chosen as a central nonlethal sampling option which allowed closed sampling for appropriate determination of venous blood gases and acid based status in the noncritical patient.9,22,23 Adverse or unusual events were recorded and records were reviewed for similar events in nonsedated birds from a previous study.19
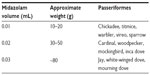  | Table 1 Midazolam (5 mg/mL solution) dosing for field sedation of Passeriformes |
  | Table 2 Modified sedation scale and clinical effect of midazolam on free-living Passeriformes |
Sample analysis
Blood samples were collected with needle and syringe and then transferred to lithium heparin microtainer tubes (Sarstedt, IDEXX Laboratories, Westbrook, ME, USA); analyses occurred within 5 minutes of sample collection using the i-STAT® system (Abbott Laboratories, Abbott Park, IL, USA). Blood gas cartridges (CG4+ or CG8+) were used first, followed by cartridges which did not analyze blood gases (6+, E3+, or E3+Cl). Time of day was recorded as time of analyte results for the first cartridge. Analytes determined were based on the cartridge and blood volume obtained and included: pH (venous), venous carbon dioxide partial pressure (pCO2), venous oxygen partial pressure (pO2), lactate, bicarbonate (HCO3), total carbon dioxide (TCO2), base excess (BE), venous dissolved oxygen (sO2), ionized calcium, glucose, blood urea nitrogen (BUN), calculated hematocrit (Hct), hemoglobin (Hgb), sodium (Na), potassium (K), and chloride (Cl). Most values obtained by the i-STAT® system are measured directly, but TCO2, BE, Hgb and sO2, are calculated.24 Lithium heparin microhematocrit tubes (Statspin® centrifuge; Iris® sample processing, Westwood, MA, USA) were used to determine packed cell volume (PCV) and were centrifuged at 15,000 g for 3 minutes within 24 hours of blood collection. After venipuncture, a physical examination was performed. Birds were then humanely killed via thoracic compression and frozen until preparation as standard museum specimens that are deposited at the Biodiversity Research and Teaching Collections at Texas A&M University. Species, sex, and age were determined based on external field markings and confirmed during specimen preparation. Body weight was determined at specimen preparation.
Additional calculations
Temperature corrections with an assumed Passeriforme core body temperature of 41.3°C were performed for pH, pCO2, and pO2 as follows:9,24
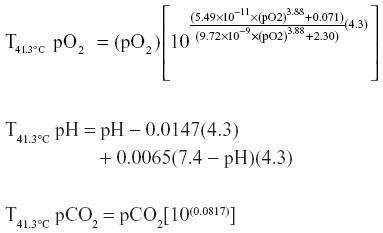
Anion gap was calculated for the median analyte values of birds treated and not treated with midazolam via the formula:
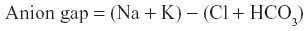
For comparison to Hgb values determined by the iSTAT-1®, Hgb was calculated via the passerine formula:25
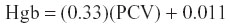
Statistical methods
Initial data analyses were performed with the statistical software Analyse-it® (Analyse-it Software, Ltd., Leeds, UK) for Microsoft Excel (Version 2.20 Microsoft Office v 2010; Microsoft Corporation, Redmond, WA, USA). To assess the effect of midazolam on blood analytes, analyte values of Passeriformes receiving midazolam were compared with those obtained from the same species of passerines collected in the previous years which did not receive midazolam.19 Species were grouped for comparison of values via Kruskal–Wallis one-way analysis of variance when species numbers were greater than two and had at least one bird in the sedated and nonsedated categories (Table 3). Analysis of variance was used to assess the effect of midazolam dose (mg/kg) on bird sedation score. Kruskal–Wallis one-way analysis of variance assessed the effect of Hct on BUN. A Bland–Altman plot was used to determine the agreement of Hct and PCV for all samples. Additional statistical analysis was performed with R for mixed modeling.26 The effect of selected variables on iSTAT-1 values was assessed using a linear mixed model with iSTAT-1 values as outcome variables; age, sex, midazolam sedation (1 or 0), and interactions as fixed effects; and species as random effect. Residual plots were used to assess linearity, homogeneity of variances, normality, and outliers. A type III analysis of variance was performed on the fixed effects and post hoc comparisons were performed using a Tukey adjustment. When necessary to meet assumptions of the linear mixed models, log transformation of the outcome variables was used. When assumptions of the linear models could not be met, a two-sample Wilcoxon test was performed on the iSTAT-1 values to assess the effect of midazolam sedation, sex, and age separately. Alpha of 0.05 was used for significance for all statistical outcomes.
Results
One hundred and four birds were captured and sedated from a variety of Passeriformes, mostly of the Paridae family (Table 3). Midazolam doses administered averaged 5.6±2.7 mg/kg. Notable side effects were regurgitation in two birds and egg production in three birds after administration of midazolam; these clinical signs were not noted in the previous field season. A single bird, excluded from the figure, received 2.79 mg/kg midazolam and was given a sedation score of 2 but died during sampling. Sedation scores increased as midazolam dose increased (Figure 1). To assess the effect of midazolam on multiple blood analytes, we compared analytes of blood samples from 95 Passeriformes which had midazolam administered to those obtained from 96 birds which were not administered midazolam (Table 3). Electrolyte concentrations and venous blood gas analytes were affected by midazolam sedation although hematological values (PCV, Hgb, Hct) and pH were not (Tables 3–5). The midazolam-treated group had lower pO2 and sO2 while pCO2, TCO2, and HCO3 were higher in birds receiving midazolam. Modeling of multiple variables (species, age, sex, and midazolam administration) found that some variability occurred based on species for all analytes (Table 5). However, blood gases and acid–base analytes appeared least affected by species (<27%). Age affected BE and HCO3 while the effect of sex was limited to glucose, Hct, and Hgb. Most BUN values were below the limit of detection (2 mg/dL) for the analyzer (n=95); reported BUN values (n=12) were nonparametrically distributed (Shapiro–Wilk P<0.0001). Detection of BUN was not affected by Hct (Kruskal–Wallis P=0.5923) midazolam administration did not have effect on detection or detected concentrations of BUN. (Kruskal–Wallis P=0.2833) (n=60, median 5.0, 95% confidence interval 4.5–7.6 mg/dL). PCV as determined by centrifugation and Hct as reported by the iSTAT-1 had poor agreement (Figure 2). Hgb as determined by the iSTAT-1 and via calculation from PCV had poor agreement (Figure 3). Temperature correction of median values for venous blood pH (pHT41.3 =7.507), pO2, and pCO2 in groups treated and not treated with midazolam caused no clinically significant changes. Temperature correction of median pO2 venous blood values of birds receiving and not receiving midazolam, respectively, were 46.11 and 51.51 mmHg. Temperature correction of pCO2 resulted in median pCO2T41.3 with and without midazolam equaling 31.74 and 37.18 mmHg, respectively. Median anion gap calculated for groups administered and not administered midazolam also failed to reveal a clinically significant difference at 14.4 and 13.1, respectively.
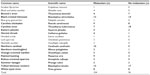  | Table 3 Midazolam treated and untreated Passeriformes sampled for iSTAT-1 values |
Discussion
Sedation
Based on adequate sedation for most birds in this study and the death of only a single bird, midazolam appears safe and efficacious with a wide margin of safety for short-term field sedation in Passeriformes. Intranasal administration was chosen based on a previous study that found that this route was easy and offered high bioavailability, rapid onset of action, reduced pain compared to intramuscular administration, and reduced likelihood of neuromuscular damage in these very small species.15 In addition, intranasal administration requires minimal training or equipment and is rapid and inexpensive.27 However, full recovery and release of birds was not a goal of this study. Further study of midazolam reversal and monitoring prior to release as well as long term effects of this sedative drug on free-living avian species are recommended. This study was similar to a previous trial which found an increased risk of regurgitation with use of this drug, as this side effect was restricted to treated birds.27 Body temperature, respiratory rate, and duration of recumbency were not assessed in this study, based on the difficulty of these assessments in field conditions and on small free-flighted subjects, but are recommended for future assessments of midazolam in birds.15,27
The dose range chosen was similar to those previously administered intranasally in other small, captive bird species such as budgerigars, finches, and canaries.14,17,28 As expected, sedation increased based on increasing doses of midazolam. However, our calculated dose range extended to above that previously reported without causing apparent immediate harm in birds given higher doses, and many birds (n=14) were unacceptably sedated using doses at or above the previously recommended ranges.12,14,15,18 Our study supports the concept that a wide therapeutic window exists in Passeriformes, as in other species, and that the standard lower dose used on larger birds may not provide sufficient sedation in some small birds. Similarly, in the short-term, a higher dose may be safe for sedation. However species effect on sedation level, if any, was not assessed or determined based on small sample sizes.
Analytes
In contrast to a single previous study of the effect of midazolam on biochemical values in birds, multiple biochemical values varied based on midazolam administration in this study.11 Glucose concentrations were higher in samples obtained from birds administered midazolam than those that were not. A sex-related effect was also further confirmed in this study as found previously.19 Mechanisms for the relative hyperglycemia in male birds and birds receiving midazolam are unclear. However, estrogens, other hormones, and sedation can affect glucose levels possibly to the significant, although nonclinically relevant, extent observed.29 Reduced struggling of the sedated bird could have lessened peripheral glucose use and metabolism of glycogen stores, resulting in increased blood glucose concentrations.
Despite low sample size, decreased BUN concentration and decreased likelihood of detecting BUN in birds receiving midazolam based on linear modeling suggests that this analyte deserves further investigation as a health indicator of Passeriformes. In birds, BUN is not the primary product of nitrogen metabolism but BUN concentration may increase with dehydration.8 However, clinical-study notes failed to reveal clinical signs of dehydration or hemoconcentration in those birds with detectable BUN.
Increased potassium in the group untreated with midazolam could be attributable to increased hemolysis, rhabdomyolysis, or other exertional trauma in the group not receiving sedation.9,11 Certainly, capture myopathy has been reported in multiple avian species; Passeriformes may also be susceptible.30–32 Dietary intake was considered an unlikely cause of this difference based on species grouping and similar times of year of sampling. Hemolysis was not assessed in this study. Intracellular concentrations of potassium in the Passeriforme erythrocyte have not been evaluated and would be an important consideration for future studies. While a variety of diseases have been associated with increased potassium concentrations, all birds sampled appeared healthy based on physical examination at the time of sampling. The lower chloride in the group administered midazolam was statistically significant but a relatively minor clinical change; no sodium change was associated with this finding. Regurgitation, egg production, and other fluid loss may have resulted based on centrally mediated muscular relaxation of midazolam in this group, resulting in lower sodium. In Passeriformes administered midazolam, one must account for expected changes in electrolyte concentrations if these analytes are under scrutiny to assess population or ecosystem health.
Lactate concentrations were lower in birds receiving midazolam, suggesting that increased exertion occurred in birds not receiving midazolam. Anxiolysis, sedation, hypnosis, or the centrally mediated muscle relaxant effects of midazolam likely mediated this result. The similar pH and anion gap of the treated and untreated groups suggests that neither midazolam nor handling without midazolam creates life-threatening uncompensated acid–base imbalance. Thus, while midazolam is safe, it is not necessary unless complicated capture or sampling is anticipated.9,11 Increased venous concentrations of HCO3 in birds treated with midazolam provides further evidence of acid–base compensation. Hypoventilation in these birds likely resulted in increased relative CO2 concentrations; bicarbonate is then increased to normalize pH; thus, a relative increase in base excess in the treated group likely occurred based on compensatory relative metabolic alkalosis.
pCO2 was higher, and sO2% and pO2 were lower, in birds administered midazolam suggesting a state of a relative hypoventilation in birds receiving this drug. These analytes were among those least affected by species, age, or sex. Hypotension and respiratory depression are common side effects of this drug in humans and may have occurred in the Passeriformes studied. Decreased respiratory rate has been reported after administration of this drug to avian species.15,27 Future studies should evaluate respiratory rate, cardiac output, and perfusion; however, some of these parameters are challenging to evaluate in a field setting.
Regardless of midazolam administration, birds maintained a relatively basic blood pH; temperature correction did not result in clinically relevant change in this value (pHT41.3 =7.507). Temperature correction of blood gas analytes were consistent with birds having a higher venous pO2 and pCO2 than humans which is similar to findings in other avian arterial blood gases.9,33–36 An attenuated increase in body temperature has been reported in parrots after administration of midazolam; thus, parameters subject to temperature correction might be expected to change less in birds given midazolam based on the moderated temperature in treated birds.15 However, cloacal temperature was not measured in treated or untreated birds based on the lack of technical feasibility.
No differences were noted for the erythrocyte parameters (PCV, Hct, Hgb) of birds that did and did not receive midazolam. Linear mixed modeling further supported that sex but not age or midazolam administration affected Hct and Hgb. PCV agreed poorly with Hct, as previously published.19 Values for Hct averaged 14.8% lower on the iSTAT-1® system than that provided via centrifugal determination of PCV. This difference is likely based on different analysis methods; the shape, size, and density of avian blood cells; and increased glucose concentrations and the lower total protein of avian blood.24,37 The iSTAT-1 system is not recommended as a sole determinant of Hgb or Hct in birds as results do not agree clinically with the gold standard PCV. Use of a single correction factor for all avian species appears inappropriate based on findings in this study and other previously published data.19,35,38
The iSTAT-1 system calculates Hgb (g/dL) as hematocrit (% PCV) ×0.34.24 This calculation, which differs from that validated for Passeriformes, and the erroneous PCV determination make it highly unlikely that Hgb determined by the iSTAT-1 is accurate. We report results here only for completeness and recommend that future studies address the validity of this unit for determination of Hgb in birds.
Conclusion
Midazolam is efficacious and has a broad therapeutic safety margin for short-term sedation of free-living Passeriformes. However, the recovery period requires more study before we can recommend this sedative for field use in birds destined for release. Hematological variables (Hct, PCV, Hgb) were minimally affected by administration of midazolam. However, multiple blood gas parameters, electrolyte concentrations, and biochemical blood analytes were affected by administration of midazolam and should be taken into consideration if used to assess avian population health. Changes in glucose, potassium, and lactate concentrations support the hypothesis that midazolam lessens the stress of free-living birds undergoing capture. However, blood gas values suggest that birds administered midazolam suffer relative hypoventilation. Further assessment of midazolam for field use, as well as the companion reversal drug flumazenil to facilitate assessment of Passeriforme population and therefore ecosystem health, is recommended.
Acknowledgments
Thanks to Schubot Exotic Bird Health Center and the FC Dezendorf Trust; New Wells Point Partners; and the Allen, Biesele, Decker, Ellis, Evancho, Fehrenkamp, Kelsay, Kensinger, Mollo, Panak, Rivera, Simons, Smith, Tyler, Verser, Wiggins, and especially Willis families for support and land access for Texas Ecolabs. This is publication number 1480 of the Texas A&M University Biodiversity Research and Teaching Collections.
Disclosure
The authors report no conflicts of interest in this work.
References
Breuer K, Lill A, Baldwin J. Hematological and Body-Mass Changes of Small Passerines Overwintering in South-Eastern Australia. Aust J Zool. 1995;43(1):31–38. | |
Booth CE, Elliott PF. Hematological responses to hematozoa in North American and neotropical songbirds. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(3):451–467. | |
Hill EF, Murray HC. Seasonal variation in diagnostic enzymes and biochemical-constituents of captive northern bobwhites and passerines. Comp Biochem Phys B. 1987;87(4):933–940. | |
Swanson DL. Seasonal Metabolic Variation in Birds: Functional and Mechanistic Correlates. In: Thompson CF, editor. Current Ornithology. Vol 17. New York: Springer; 2010:75–129. | |
Deem SL, Parker PG, Cruz MB, Merkel J, Hoeck PE. Comparison of blood values and health status of Floreana Mockingbirds (Mimus trifasciatus) on the islands of Champion and Gardner-by-Floreana, Galápagos Islands. J Wildl Dis. 2011;47(1):94–106. | |
Fokidis HB, Greiner EC, Deviche P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J Avian Biol. 2008;39(3):300–310. | |
Norte AC, Ramos JA, Sousa JP, Sheldon BC. Variation of adult Great Tit Parus major body condition and blood parameters in relation to sex, age, year and season. J Ornithol. 2009;150(3):651–660. | |
Bairlein F, Totzke U. New aspects on migratory physiology of trans-Saharan passerine migrants. Ornis Scandinavica. 1992;23(3):244–250. | |
Harms CA, Harms RV. Venous blood gas and lactate values of mourning doves (Zenaida macroura), boat-tailed grackles (Quiscalus major), and house sparrows (Passer domesticus) after capture by mist net, banding, and venipuncture. J Zoo Wildl Med. 2012;43(1):77–84. | |
Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–365. | |
Ward JM, Gartrell BD, Conklin JR, Battley PF. Midazolam as an adjunctive therapy for capture myopathy in Bar-tailed Godwits (Limosa lapponica baueri) with prognostic indicators. J Wildl Dis. 2011;47(4):925–935. | |
Mans C, Guzman DS, Lahner LL, Paul-Murphy J, Sladky KK. Sedation and physiologic response to manual restraint after intranasal administration of midazolam in Hispaniolan Amazon parrots (Amazona ventralis). J Avian Med Surg. 2012;26(3):130–139. | |
Vesal N, Eskandari MH. Sedative effects of midazolam and xylazine with or without ketamine and detomidine alone following intranasal administration in Ring-necked Parakeets. J Am Vet Med Assoc. 2006;228(3):383–388. | |
Vesal N, Zare P. Clinical evaluation of intranasal benzodiazepines, alpha-agonists and their antagonists in canaries. Vet Anaesth Analg. 2006;33(3):143–148. | |
Mans C, Braun J. Intranasal Sedation with Midazolam or Midazolam Butorphanol in Psttacine Birds: A Retrospective Review of 114 Cases. Paper presented at: 1st International Conference on Avian, Herpetological and Exotic Mammal Medicine; April 20–26, 2013; Wiesbaden. | |
Day TK, Roge CK. Evaluation of sedation in quail induced by use of midazolam and reversed by use of flumazenil. J Am Vet Med Assoc. 1996;209(5):969–971. | |
Bigham AS, Zamani Moghaddam AK. Finch (Taeneopygia guttata) sedation with intranasal administration of diazepam, midazolam or xylazine. J Vet Pharmacol Ther. 2013;36(1):102–104. | |
Valverde A, Honeyman VL, Dyson DH, Valliant AE. Determination of a sedative dose and influence of midazolam on cardiopulmonary function in Canada geese. Am J Vet Res. 1990;51(7):1071–1074. | |
Heatley JJ, Carey J, Russell KE, Voelker G. Clinicopathologic analysis of Passeriforme venous blood reflects transitions in elevation and habitat. Veterinary Medicine: Research and Reports. 2013;2013(4):21–29. | |
Karampour A, Nikahval B, Sarchahi AA, Vesal N. Clinical evaluation of the sedative properties of acepromazine-xylazine combinations with or without atropine and their effects on physiologic values in dogs. Vet Arh. 2011;81(4):485–498. | |
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. | |
Kelly AM. Review article: Can venous blood gas analysis replace arterial in emergency medical care. Emerg Med Australas. 2010;22(6):493–498. | |
Kirubakaran C, Gnananayagam JE, Sundaravalli EK. Comparison of blood gas values in arterial and venous blood. Indian J Pediatr. 2003;70(10):781–785. | |
Abbott Point of Care: iSTAT-1 system manual [webpage on the Internet]. Abbot Park: Abbot Laboratories; 2012 [updated May 2014]. Available from: http://www.abbottpointofcare.com/Customer-Info-Center/User-Documentation.aspx. Accessed October 1, 2012. | |
Velguth KE, ME PE, Hoover JP. Relationship of hemoglobin concentration to packed cell volume in avian blood samples. J Avian Med Surg. 2010;24(2):115–121. | |
The R project for statistical computing [webpage on the Internet]. Available at http://www.r-project.org/. Accessed November 18, 2014. | |
Ajadi RA, Kasali OB, Makinde AF, Adeleye AI, Oyewusi JA, Akintunde OG. Effects of midazolam on ketamine-xylazine anesthesia in guinea fowl (Numida meleagris galeata). J Avian Med Surg. 2009;23(3):199–204. | |
Sadegh AB. Comparison of intranasal administration of xylazine, diazepam, and midazolam in budgerigars (Melopsittacus undulatus):clinical evaluation. J Zoo Wildl Med. 2013;44(2):241–244. | |
Goldner MG. Oral hypoglycemic agents past and present other than sulfonylurea compounds. AMA Arch Intern Med. 1958;102(5):830–840. | |
Ruder MG, Noel BL, Bednarz JC, Keel MK. Exertional myopathy in pileated woodpeckers (Dryocopus pileatus) subsequent to capture. J Wildl Dis. 2012;48(2):514–516. | |
Marco I, Mentaberre G, Ponjoan A, Bota G, Mañosa S, Lavin S. Capture myopathy in little bustards after trapping and marking. J Wildl Dis. 2006;42(4):889–891. | |
Dabbert CB, Powell KC. Serum enzymes as indicators of capture myopathy in mallards (Anas platyrhynchos). J Wildl Dis. 1993;29(2):304–309. | |
Martin MP, Wineland M, Barnes HJ. Selected blood chemistry and gas reference ranges for broiler breeders using the i-STAT handheld clinical analyzer. Avian Dis. 2010;54(3):1016–1020. | |
Paula VV, Fantoni DT, Otsuki DA, Auler JOC Jr. Blood-gas and electrolyte values for Amazon parrots (Amazona aestiva). Pesquisa Vet Brasil. 2008;28(2):108–112. | |
Rettenmund C, Heatley J, Russell K. Comparison of Two Analyzers in determining selected Electrolyte, Acid-Base, and Venous Blood Gas parameters of Quaker Parrots (Myiopsitta monachus). Paper presented at: Association of Avian Veterinarians 32nd Annual Conference and Expo with the Association of Exotic Mammals Veterinarians; August 6–12, 2011; Seattle, WA. | |
Steinmetz HW, Vogt R, Kästner S, Riond B, Hatt JM. Evaluation of the i-STAT portable clinical analyzer in chickens (Gallus gallus). J Vet Diagn Invest. 2007;19(4):382–388. | |
Guevara E, Gonzalez F. Prediction of glucose concentration by impedance phase measurements. AIP Conf Proc. 2008;1032:259–261. | |
Heatley J, Russel K, Norby B, Brightsmith D. Electrolytes, pH, and ionized calcium as health indicators in free living nestling macaws. Paper presented at: American Association of Zoo Veterinarians and American Association of Wildlife Veterinarians; October 25–30, 2009; Tulsa, OK. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





