Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 7
Management of adult diabetic ketoacidosis
Authors Gosmanov AR, Gosmanova E, Dillard-Cannon E
Received 18 April 2014
Accepted for publication 13 May 2014
Published 30 June 2014 Volume 2014:7 Pages 255—264
DOI https://doi.org/10.2147/DMSO.S50516
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Aidar R Gosmanov,1 Elvira O Gosmanova,2 Erika Dillard-Cannon3
1Division of Endocrinology, Diabetes and Metabolism, 2Division of Nephrology, Department of Medicine, 3Department of Microbiology, Immunology, and Biochemistry, University of Tennessee Health Science Center, Memphis, TN, USA
Abstract: Diabetic ketoacidosis (DKA) is a rare yet potentially fatal hyperglycemic crisis that can occur in patients with both type 1 and 2 diabetes mellitus. Due to its increasing incidence and economic impact related to the treatment and associated morbidity, effective management and prevention is key. Elements of management include making the appropriate diagnosis using current laboratory tools and clinical criteria and coordinating fluid resuscitation, insulin therapy, and electrolyte replacement through feedback obtained from timely patient monitoring and knowledge of resolution criteria. In addition, awareness of special populations such as patients with renal disease presenting with DKA is important. During the DKA therapy, complications may arise and appropriate strategies to prevent these complications are required. DKA prevention strategies including patient and provider education are important. This review aims to provide a brief overview of DKA from its pathophysiology to clinical presentation with in depth focus on up-to-date therapeutic management.
Keywords: DKA treatment, insulin, prevention, ESKD
Letter about this article has been published
Introduction
In 2009, there were 140,000 hospitalizations for diabetic ketoacidosis (DKA) with an average length of stay of 3.4 days.1 The direct and indirect annual cost of DKA hospitalizations is 2.4 billion US dollars. Omission of insulin is the most common precipitant of DKA.2,3 Infections, acute medical illnesses involving the cardiovascular system (myocardial infarction, stroke) and gastrointestinal tract (bleeding, pancreatitis), diseases of the endocrine axis (acromegaly, Cushing’s syndrome), and stress of recent surgical procedures can contribute to the development of DKA by causing dehydration, increase in insulin counter-regulatory hormones, and worsening of peripheral insulin resistance. Medications such as diuretics, beta-blockers, corticosteroids, antipsychotics, and/or anticonvulsants may affect carbohydrate metabolism and volume status and, therefore, could precipitate DKA. Other factors that may contribute to DKA include psychological problems, eating disorders, insulin pump malfunction, and illegal substance use.4,5 It is now recognized that new-onset type 2 diabetes mellitus can manifest with DKA.6 These patients are obese, mostly African Americans or Hispanics, and extremely insulin resistant on presentation.7
Pathophysiology
Insulin deficiency, increased insulin counter-regulatory hormones (cortisol, glucagon, growth hormone, and catecholamines), and peripheral insulin resistance lead to hyperglycemia, dehydration, ketosis, and electrolyte imbalance, which underlie the pathophysiology of DKA.8 Due to increased lipolysis and decreased lipogenesis, abundant free fatty acids are converted to ketone bodies: β-hydroxybutyrate (β-OHB) and acetoacetate. Hyperglycemia-induced osmotic diuresis, if not accompanied by sufficient oral fluid intake, leads to dehydration, hyperosmolarity, electrolyte loss, and subsequent decrease in glomerular filtration rate. With decline in a renal function, glycosuria diminishes and hyperglycemia worsens. With impaired insulin action and hyperosmolar hyperglycemia, potassium uptake by skeletal muscle is markedly diminished; also hyperosmolarity can cause efflux of potassium from cells. This results in intracellular potassium depletion and subsequent loss of potassium via osmotic diuresis, causing reduction of total body potassium averaging 3–5 mmol/kg of body weight. Nevertheless, DKA patients can present with a broad range of serum potassium concentrations. A “normal” plasma potassium concentration still indicates that total body potassium stores are severely diminished, and the institution of insulin therapy and correction of hyperglycemia will result in hypokalemia. On average, patients with DKA may have the following deficit of water and key electrolytes per kg of body weight: free water 100 mL/kg; sodium 7–10 mEq/kg; potassium 3–5 mEq/kg; chloride 3–5 mmol/kg; and phosphorus 1 mmol/kg.3
Diagnosis
Diagnostic criteria for DKA include presence of blood glucose >250 mg/dL, arterial pH of ≤7.30, bicarbonate level of ≤18 mEq/L, and adjusted for albumin anion gap of >10–12.3 Positive serum and urine ketones may further support the diagnosis of DKA. In early DKA, acetoacetate concentration is low, but it is a major substrate for ketone measurement by many laboratories; therefore, ketone measurement in serum by usual laboratory techniques has a high specificity but low sensitivity for the diagnosis of DKA. Conversely, β-OHB is an early and abundant ketoacid, which may first signal the development of DKA; however, its determination requires use of a specific assay that is different from those used for standard ketone bodies measurement. The levels of β-OHB of ≥3.8 mmol/L measured by a specific assay were shown to be highly sensitive and specific for DKA diagnosis.9 In patients with chronic kidney disease stage 4–5, the diagnosis of DKA could be challenging due to the presence of concomitant underlying chronic metabolic acidosis or mixed acid-base disorders. Anion gap of >20 usually supports the diagnosis of DKA in these patients.2
Treatment
The therapeutic goals of DKA management include optimization of 1) volume status; 2) hyperglycemia and ketoacidosis; 3) electrolyte abnormalities; and 4) potential precipitating factors. The majority of patients with DKA present to the emergency room. Therefore, emergency physicians should initiate the management of hyperglycemic crisis while a physical examination is performed, basic metabolic parameters are obtained, and final diagnosis is made. Several important steps should be followed in the early stages of DKA management:
- collect blood for metabolic profile before initiation of intravenous fluids;
- infuse 1 L of 0.9% sodium chloride over 1 hour after drawing initial blood samples;
- ensure potassium level of >3.3 mEq/L before initiation of insulin therapy (supplement potassium intravenously if needed);
- initiate insulin therapy only when steps 1–3 are executed.
The protocol for the management of patients with DKA is presented in Figure 1. It must be emphasized that successful treatment requires frequent monitoring of clinical and metabolic parameters that support resolution of DKA (Table 1).
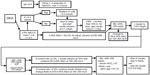  | Figure 1 Workflow of management of adult DKA. |
  | Table 1 Checklist of DKA management milestones |
Fluid therapy
Fluid loss averages approximately 6–9 L in DKA. The goal is to replace the total volume loss within 24–36 hours with 50% of resuscitation fluid being administered during the first 8–12 hours. A crystalloid fluid is the initial fluid of choice.10 Current recommendations are to initiate restoration of volume loss with boluses of isotonic saline (0.9% NaCl) intravenously based on the patient’s hemodynamic status.3 Thereafter, intravenous infusion of 0.45% NaCl solution based on corrected serum sodium concentration will provide further reduction in plasma osmolality and help water to move into the intracellular compartment. Hyperosmolar hyponatremia due to hyperglycemia is a frequent laboratory finding in DKA and is usually associated with dehydration and elevated corrected sodium concentrations.
The optimal rate of initial fluid administration was addressed in a prospective randomized controlled study in which patients were treated with either 500 mL/hour (h) or 1 L/h of isotonic fluid. There were no differences in the resolution of DKA, mortality, or complications.11 Most protocols call for an initial bolus of isotonic crystalloid solution (0.9% saline) at a starting rate of 15–20 mL/kg/h (1–1.5 L/h) for the first hour.3,8 Following the initial hydration, fluids can be administered at a decreased rate of 4–14 mL/kg/h. Tonicity of subsequent solution is dependent upon hydration status, electrolyte balance, and urine output. Rapid correction of serum sodium and, hence, serum osmolality by hypotonic fluids may carry an increased risk of cerebral edema. On the other hand, continuous isotonic fluid therapy in pediatric patients was found to have an increased risk of a non-anion gap hyperchloremic acidosis possibly leading to longer hospital stays due to erroneous diagnosis of persistent ketoacidosis.12 Accordingly, safe practice of fluid resuscitation in DKA patients includes provision of initial bolus of isotonic saline at 15–20 mL/kg/h followed by hypotonic saline solution (0.45% saline) at a rate of 4–14 mL/kg/h as long as the patient is hemodynamically stable and corrected serum sodium is normal to high. If a patient becomes hyponatremic based on corrected serum sodium, initiation of 0.9% saline at a rate of 150–250 mL/h is recommended until eunatremia is achieved.3 Replacement of the water deficit using high rates of intravenous fluids has not been studied in pediatric patient populations and, therefore, this approach cannot be recommended for the management of pediatric DKA.
Intravascular and extravascular volume resuscitation will decrease hyperglycemia by stimulating osmotic diuresis if renal function is not severely compromised and enhance peripheral action of insulin (insulin effects on glucose transport are decreased by hyperglycemia and hyperosmolarity). When glucose levels fall below 200–250 mg/dL, intravenous fluids should be switched to dextrose-containing 0.45% NaCl solution to prevent hypoglycemia, and/or insulin infusion rate should be decreased. Special considerations should be given to patients with congestive heart failure and chronic kidney disease. These patients tend to retain fluids; therefore, caution should be exercised during volume resuscitation in these patient groups. Urine output monitoring is an important step in patients with hyperglycemic crises.
Insulin therapy
Treatment of DKA with intravenous insulin
Insulin administration is essential in DKA treatment because it promotes glucose utilization by peripheral tissues, diminishes glycogenolysis and gluconeogenesis, and suppresses ketogenesis. Intravenous infusion is a preferred route of insulin delivery in patients with DKA.13 Insulin infusion without initial volume resuscitation is not advised as it may only worsen dehydration. Insulin treatment has evolved from the use of high-dose insulin, with doses up to 100 U/h by various routes of administration, to lower doses in the range of 5–10 U/h.14 We recommend an initial bolus of regular insulin of 0.1 U/kg followed by continuous insulin infusion. If plasma glucose does not fall by at least 10% in the first hour of insulin infusion rate, 0.1 U/kg bolus of insulin can be given once more while continuing insulin infusion.3 Of clinical significance is the phenomenon of hyperglycemia-induced insulin resistance; with reduction of glycemia, there can be a nonlinear decrease in insulin requirements. When plasma glucose reaches 200–250 mg/dL, the insulin rate can be decreased by 50% or to the rate of 0.02–0.05 U/kg/h (Figure 1).
Clinical importance of the initial insulin bolus in the insulin management of DKA has been recently challenged in a study that compared efficacy and safety of two strategies of insulin infusion – with and without priming bolus.15 The authors found that there were no differences in outcomes between a group of patients who were treated with the infusion of regular insulin at a dose of 0.14 U/kg/h without administration of initial insulin bolus and a group of patients who were managed by the administration of priming insulin bolus of 0.07 U/kg followed by the continuous insulin infusion at 0.07 U/kg/h. The efficacy of the therapeutic approach with an insulin dose of 0.1 U/kg was not assessed in that study. A more recent study showed no significant difference in incidence of hypoglycemia, rate of glucose change or anion gap, length of stay in the emergency department, or hospital stay in patients receiving an infusion rate of 0.1 U/kg/h with or without insulin bolus.16 No previous studies have compared clinical outcomes in pediatric DKA patients treated with and without priming insulin bolus; therefore, the use of priming bolus in pediatric DKA care is not recommended. The basis for use of a priming bolus stemmed from a study in patients with hyperosmolar hyperglycemic nonketotic diabetes, which suggested that an initial bolus could help correct the relative insulin resistance of DKA.17 As such, inconsistent results may have been due to patient variables such as absence of ketosis and/or presence of severe hyperglycemia. Current American Diabetes Association recommendations suggest one of the two above options for intravenous insulin therapy (with or without insulin bolus), considering a serum potassium >3.3 mEq/L.3,18 In our opinion, the majority of patients with DKA can rapidly become insulin sensitive following the administration of intravenous fluids and improvements in hyperglycemia. Therefore, to avoid hypoglycemia and rapid shifts of glucose and water between extracellular and intracellular compartments, the larger rates of insulin infusion should be reserved for obese and more insulin-resistant DKA patients. The administration of priming insulin bolus can be feasible in less insulin-resistant DKA patients initially presenting with extreme hyperglycemia.
Treatment of DKA with subcutaneous insulin
Early investigations assessing optimal insulin doses and administration route in the treatment of DKA demonstrated that subcutaneous delivery of regular insulin is effective but inferior to the intravenous insulin infusion.13 The approval of rapid-acting insulin analogs (aspart, glulisine, and lispro) offered new paradigms in the management of diabetes mellitus, including therapy of DKA. In two prospective, randomized, open-label studies, subcutaneous insulin administration was compared to intravenous insulin infusion, which is the standard of care for patients admitted to the hospital with mild uncomplicated DKA.19,20 Subcutaneous administration of insulin aspart19 and insulin lispro20 every 1 or 2 hours was as safe and efficient as continuous insulin infusion performed in the intensive care unit (ICU). Amount of used insulin, time to DKA resolution, rate of hypoglycemia, and the length of hospital stay were similar between subcutaneous and intravenous insulin groups in both studies.19,20 Following these investigations, others demonstrated equal efficacy and safety of subcutaneous insulin lispro compared with continuous insulin infusion in patients with mild and moderate DKA.21
Recent studies have therefore clearly outlined therapeutic feasibility and cost-effectiveness of the treatment of mild uncomplicated DKA with insulin analogs outside of the ICU setting; however, the proposed subcutaneous insulin protocols are yet to find widespread support from hospital administrations and treating physicians. Lack of nursing and medical staff training, presence of multiple accompanying comorbidities in diabetes patients, insufficient resources with which to conduct frequent bedside glucose testing in hospital wards, and absence of financial incentives to treat DKA outside of ICUs are some of the factors that diminish the enthusiasm of providers to take on this important issue of health care resource utilization without compromising patient care. Future trials testing less complex subcutaneous insulin delivery protocols should be considered in an attempt to simplify management of mild DKA in a non-ICU setting.
Potassium, bicarbonate, and phosphate therapy
Serum potassium should be closely monitored during DKA treatment. Insulin administration and correction of acidemia and hyperosmolality drive potassium intracellularly, resulting in hypokalemia that may lead to arrhythmias and cardiac arrest. If serum potassium decreases to <3.3 mEq/L during DKA treatment, insulin should be stopped and potassium administered intravenously. Small amounts of potassium (20–30 mEq/L) are routinely added to intravenous fluids when serum potassium is between 3.3 and 5.3 mmol/L. No replacement is needed for potassium levels >5.3 mmol/L.
Bicarbonate therapy is not indicated in mild and moderate forms of DKA because metabolic acidosis will correct with insulin therapy.3,8 The use of bicarbonate in severe DKA is controversial due to a lack of prospective randomized studies. It is thought that the administration of bicarbonate may actually result in peripheral hypoxemia, worsening of hypokalemia, paradoxical central nervous system acidosis, cerebral edema in children and young adults, and an increase in intracellular acidosis. Because severe acidosis is associated with worse clinical outcomes and can lead to impairment in sensorium and deterioration of myocardial contractility, bicarbonate therapy may be indicated if the pH is 6.9 or less. Therefore, the infusion of 100 mmol (two ampoules) of bicarbonate in 400 mL of sterile water mixed with 20 mEq potassium chloride over 2 hours, and repeating the infusion until the pH is greater than 7.0, could be recommended pending the results of future randomized controlled trials.
A whole-body phosphate deficit in DKA can average 1 mmol/kg. Insulin therapy during DKA will further lower serum phosphate concentration; 90% of patients were shown to have developed hypophosphatemia during infusion of insulin and fluids.22 Prospective randomized studies have failed to show any beneficial effect of phosphate replacement on the clinical outcomes in DKA. Phosphate replacement has actually been implicated in creating a state of severe hypocalcemia;23,24 however, careful phosphate replacement is indicated in patients with a serum phosphate concentration less than 1.0 mg/dL or in patients with a serum phosphate level between 1.0 and 2.0 mg/dL and cardiac dysfunction, anemia, or respiratory depression. Initial replacement strategy may include infusion of potassium phosphate at the rate of 0.1–0.2 mmol/kg over 6 hours, depending on the degree of phosphate deficit (10 mL of potassium phosphate solution for intravenous use contains 30 mmol of phosphorous and 44 mmol of potassium). Overzealous phosphate replacement may result in hypocalcemia; therefore, close monitoring of both phosphorous and calcium levels is recommended.3,23,24 Patients who have renal insufficiency and/or hypocalcemia may need less aggressive phosphate replacement.
Treatment of DKA in dialysis patients
Diabetes is a leading cause of end-stage kidney disease (ESKD) in the US.25 DKA is infrequent in dialysis patients but it has been increasingly encountered due to rising prevalence of diabetic ESKD. The kidney plays an important role in glucose homeostasis, and the loss of kidney function is often associated with improved glycemic control due to the reduction of kidney gluconeogenesis, improved insulin sensitivity with regular dialysis, and reduced insulin clearance.26 However, these processes also place ESKD patients with diabetes at higher risk of hypoglycemia.
DKA in dialysis patients may differ in the clinical and laboratory presentation and the treatment, compared with DKA in non-dialysis patients.27 Metabolic acidosis is usually present in dialysis-associated DKA. The average reported serum bicarbonate and anion gap in dialysis-dependent patients with DKA were 12.0±4.6 mmol/L and 27.2±6.4 mEq/L, respectively.28 Rarely, metabolic acidosis can be masked by concomitant metabolic alkalosis from exposure to a high bicarbonate dialysate during hemodialysis. Mixed acid base disorder in this case can produce normal or minimally reduced serum bicarbonate; nevertheless, a high anion gap will be present in DKA and serves as a clue for DKA.29 Additionally, anion gap in ESKD patients can be elevated in the absence of DKA because of accumulation of organic acids and reduced acid secretion from kidney failure.30 However, anion gap of more than 20 mEq/L is not typical for ESKD alone,31 and should prompt the search for additional causes of anion-gap metabolic acidosis, such as DKA.
It is important to note that no prospective studies have systematically evaluated strategies assessing treatment and resolution of DKA in dialysis patients; therefore, the diagnosis of DKA and monitoring for its resolution should be done in close collaboration with a nephrologist. Insulin administration is a mainstay and frequently the only treatment required for DKA management in dialysis patients.28 In the absence of data from prospective studies, the initial rate of intravenous insulin administration for dialysis patients should be similar to non-dialysis individuals, with a recommended pace of serum glucose decline of 100–125 mg/dL/h to avoid neurologic consequences from a rapid reduction in serum tonicity and intracellular swelling. In our opinion, initial insulin bolus to 0.1 U/kg followed by continuous insulin infusion at 0.05 U/kg/h may be appropriate to avoid too rapid glucose correction.
Clinically, dialysis patients usually present with minimal or no signs of volume depletion, and often have signs of extracellular volume expansion such as lower extremity and pulmonary edema and elevated blood pressure.27 The absence of volume depletion is explained by the lack of osmotic diuresis when residual renal function is severely reduced or absent. It is believed that, in dialysis patients with DKA, overall intracellular volume is preserved and extracellular volume remains normal to increased.27 Therefore, ESKD patients often do not require intravenous fluids in the absence of clinical history of extracellular fluid loss such as vomiting, diarrhea, or excessive insensible losses. If evidence for intravascular volume depletion is present, we suggest judicious administration of small boluses of normal saline (250 mL) with close monitoring of respiratory and hemodynamic parameters. Infrequently, severe DKA can lead to pulmonary edema due to hyperglycemia-associated interstitial hypertonicity.32 Pulmonary edema in DKA usually responds to administration of insulin alone;27,32 in severe cases, acute dialysis may be required.
Reduced glomerular filtration rate, insulinopenia, and hypertonicity result in positive potassium balance, placing ESKD patients with DKA at high risk for hyperkalemia.33 Hyperkalemia is typically more severe in dialysis patients compared with non-dialysis patients for the same levels of hyperglycemia and can be life threatening.33,34 Consequently, routine potassium replacement is not indicated unless plasma potassium level is below 3.3 mmol/L. Insulin is typically the only treatment necessary for hyperkalemia in dialysis-dependent patients with DKA. Severe hyperkalemia requires electrocardiographic monitoring for signs of cardiac toxicity. Potassium measurement level should be repeated 2 hours post-procedure to monitor for its intracellular rebound after hemodialysis.
The role of hemodialysis in the treatment of DKA is controversial and has not been systematically studied in ESKD patients. Severe pulmonary edema and hyperkalemia are two main indications for acute hemodialysis in DKA. Emergent dialysis in patients with DKA without clear indications can cause rapid decrease of serum tonicity, which can present a potential concern for neurological complications in ESKD patients.
Metabolic treatment targets
Serial measurements (every 2–4 hours) of metabolic parameters are required to monitor therapy and then confirm resolution of DKA. DKA is resolved when 1) plasma glucose is <200–250 mg/dL; 2) serum bicarbonate concentration is ≥15 mEq/L; 3) venous blood pH is >7.3; and 4) anion gap is ≤12. In general, resolution of hyperglycemia, normalization of bicarbonate level, and closure of anion gap is sufficient to stop insulin infusion. Anion gap is calculated by subtracting the sum of chloride and bicarbonate from measured (not corrected) sodium concentration. It can improve even before the restoration of serum bicarbonate due to hyperchloremia from normal saline infusion. Venous pH is adequate to assess the degree of acidosis with consideration that it is 0.02–0.03 lower than arterial blood. If plasma glucose is <200 mg/dL but bicarbonate and pH are not normalized, insulin infusion must be continued and dextrose-containing intravenous fluids started. The latter approach will continue to suppress ketogenesis while preventing hypoglycemia.
Insulin therapy after resolution of DKA
When the patient is able to tolerate oral intake and DKA is resolved, transition to subcutaneous insulin must be initiated. It is common to see transition from intravenous to subcutaneous insulin using sliding scale insulin only. This strategy as a sole approach should be discouraged, as it cannot provide the necessary insulin requirement in patients recovering from hyperglycemic crisis and β-cell failure. Patients should be given intermediate (neutral protamine Hagedorn) or long-acting insulin (detemir or glargine) 2 hours before termination of intravenous insulin to allow sufficient time for the injected insulin to start working (Table 2). It is also feasible to begin administration of long-acting insulin while the patient continues to receive intravenous insulin therapy. In one prospective randomized study, insulin glargine was given at a dose of 0.25 U/kg to subjects with severe hyperglycemia, including patients with DKA, within 12 hours after initiation of intravenous insulin.35 The authors found that once-daily subcutaneous insulin glargine administered during intravenous insulin infusion prevents future rebound hyperglycemia without an increased risk of hypoglycemia.
  | Table 2 Pharmacokinetics and pharmacodynamics of subcutaneous insulin preparations |
When a patient resumes oral intake, we recommend the addition of short-acting insulin for prandial glycemic coverage (Table 2). The regimen containing both long-acting and short-acting insulin is called a basal-bolus insulin regimen; it provides physiological replacement of insulin. If a patient used insulin prior to admission, the same dose can be restarted in the hospital. Insulin-naïve patients require insulin at a total dose of 0.5–0.8 U/kg/day divided as 50% basal insulin and 50% prandial insulin before each meal. Upon DKA resolution, given the possibility of fluctuating oral intake, use of once-daily long-acting insulin glargine or detemir to provide basal insulin coverage is encouraged. For those patients who are not able to adhere to or afford multiple daily insulin injections, conversion of an inpatient basal-bolus insulin regimen before discharge to a premixed insulin preparation twice a day that contains a mixture of intermediate-acting insulin neutral protamine Hagedorn and short-acting insulin formulations such as regular (Humulin R, Novolin R), aspart (NovoLog), or lispro (Humalog) can be considered. Finger-stick glucose measurements before each meal and at night should be done after discontinuation of intravenous insulin to correct for possible fluctuations in insulin needs while in the hospital.
Key DKA management points
- Start intravenous fluids before insulin therapy.
- Potassium level should be >3.3 mEq/L before the initiation of insulin therapy (supplement potassium intravenously if needed).
- Administer priming insulin bolus at 0.1 U/kg and initiate continuous insulin infusion at 0.1 U/kg/h. Measure bedside glucose every 1 hour to adjust the insulin infusion rate.
- Avoid hypoglycemia during the insulin infusion by initiating dextrose-containing fluids and/or reduction of insulin infusion rate until DKA is resolved.
- Transition to subcutaneous insulin only when DKA resolution is established.
DKA management protocols in clinical care
With a number of DKA guidelines and technical reviews in the field published by several societies,3,8,36,37 there is a call to efficiently deliver current knowledge to the bedside. One approach to delivering best clinical practices is development of inpatient standardized protocols for DKA management. Studies have shown that protocol-directed care of patients with hyperglycemic crises is both safe and efficient, as highlighted by significant decreases in length of stay without increases in the rate of iatrogenic complications.38,39 Recently, the efficacy and safety of a DKA protocol based on the 2009 American Diabetes Association consensus statement was evaluated in a retrospective review at a university teaching hospital in the US. Patients treated under this protocol experienced a decrease in time to resolution of ~10 hours without increased rates of iatrogenic hypoglycemia or hypokalemia.40 The time to DKA resolution using the universal protocol in that study was similar to the time to attaining metabolic control achieved in randomized controlled trials, which used experienced research nursing staff in the care of study patients with DKA.19,20
Outcomes of protocol-based care have differed in other institutions. One retrospective case review study conducted in the United Kingdom revealed that, though providers were aware of the existence of a universal protocol, this did not translate into protocol adherence for a number of reasons, including patient- and clinician-related factors. The greatest benefit was observed during the first few hours of early DKA management, where intravenous access was gained appropriately, initial fluid resuscitation was started, and initial laboratory testing was completed. Following early care, variations in adherence to protocol increased, as less than one-half of patients received appropriate fluid therapy or repeat laboratory studies or were referred to the appropriate care unit.41 Other studies have also demonstrated suboptimal care as a result of low adherence stemming from discontinuity of medical care, understaffing, and low experience in the care of DKA patients.42,43 Therefore, there is a need for ongoing medical staff education and training in order to increase protocol adherence throughout DKA. The care of patients with DKA should be a collaborative effort that includes the expertise of endocrinology, intensive care, medical pharmacy, and nursing specialists.
Complications
Hypoglycemia is the most frequent complication of DKA and can be prevented by timely adjustment of insulin dose and frequent monitoring of blood glucose levels. Hypoglycemia is defined as any blood glucose level below 70 mg/dL. If DKA is not resolved and blood glucose level is below 200–250 mg/dL, decrease in insulin infusion rate and/or addition of 5% or 10% dextrose to current intravenous fluids can be implemented (Figure 1). When DKA is resolved, strategies to manage hypoglycemia will depend on whether or not the patient is able to maintain oral intake. For patients who are able to drink or eat, ingestion of 15–20 g of carbohydrates, eg, four glucose tablets, 6 ounces of orange or apple juice, or “regular” soda, is advised. In patients who are nil by mouth, unable to swallow, or have an altered level of consciousness, administration of 25 mL of 50% dextrose intravenously or 1 mg glucagon intramuscularly, if no intravenous access is present, is recommended. Blood glucose should be rechecked after 15 minutes; only if the glucose level is <70 mg/dL should the above steps be repeated.
Non-anion gap hyperchloremic metabolic acidosis frequently develops during DKA treatment and is believed to occur due to urinary losses of ketoanions, which are needed for bicarbonate regeneration, and preferential reabsorption of chloride in proximal renal tubule secondary to intensive administration of chloride-containing fluids. This acidosis usually resolves spontaneously in a few days and should not affect the treatment course. Cerebral edema due to rapid reduction in serum osmolality has been reported in young adult patients.4 This condition is manifested by appearance of headache, lethargy, papillary changes, or seizures, with mortality rates reaching 70%. Mannitol infusion and mechanical ventilation should be used to treat this condition. Rhabdomyolysis is another possible complication due to hyperosmolality and hypoperfusion. Pulmonary edema can develop from excessive fluid replacement in patients with chronic kidney disease or congestive heart failure.
Prevention
Discharge planning should include diabetes education, selection of an appropriate insulin regimen that is understood by and affordable for the patient, and preparation of supplies for the initial insulin administration at home. Many cases of DKA can be prevented by better access to medical care, proper education, and effective communication with a health care provider during an intercurrent illness. Sick-day management should be reviewed with all patients and include specific information on 1) when to contact the health care provider, 2) blood glucose goals and the use of supplemental short-acting insulin during illness, 3) insulin use during fever and infection, and 4) initiation of an easily digestible liquid diet containing carbohydrates and electrolytes. Most importantly, the patient should be advised to never discontinue insulin and to seek professional advice early in the course of the illness.
Involvement of family members/caregivers when appropriate should be encouraged. They need to be educated on insulin regimen and how to perform measurements of blood glucose and β-OHB using point-of-care devices when blood glucose is >300 mg/dL. Also, a written care plan should be provided to the patient and/or caregiver, as this enhances understanding and emphasizes the importance of self-management of diabetes. Advances in technology have provided more efficient means of monitoring diabetes and maintaining glycemic control in an outpatient setting. The use of real-time continuous glucose monitoring in adult patients with type 1 diabetes has been shown to significantly lower hemoglobin A1c. Real-time continuous glucose monitoring also has the advantage of signaling to patients the early detection of glucose abnormalities, allowing for prompt intervention.44,45 At-home use of ketone meters that detect blood β-OHB has also been shown to aid in early detection and management of ketosis, which may decrease the need for specialized care. β-OHB generally does not reach levels >1.0 mmol/L outside of metabolic instability; therefore, when β-OHB levels of 1.1–3.0 mmol/L are detected, additional short-acting insulin can be administered with fluids early on to prevent DKA.46
Conclusion
Pathophysiology-driven DKA management is complex and requires careful selection of approaches aimed at restoring deficiencies in insulin, fluids, and electrolytes. Available clinical practice recommendations and guidelines offer solid foundations for achieving successful DKA resolution. However, we advise that individualized decisions should be made, as DKA patients may have unique clinical and biochemical characteristics. Safe strategies to restore volume deficit and replace insulin should be implemented, with frequent evaluations of the patient’s status aimed at monitoring for DKA resolution and avoiding potential complications. Recent studies showing clinical benefits and safety of subcutaneous insulin administration in patients with mild DKA and utility of protocol-driven care offer new pathways to reducing the cost of DKA care while maintaining quality of clinical outcomes. Also, resources should be directed toward the education of primary care providers and patients and their families so that they can identify signs and symptoms of uncontrolled diabetes earlier. With the increasing focus on health disparities, access to medical care is a major focus in determining better care in diabetes, which would ultimately contribute to decreasing the occurrence of hyperglycemic crises of diabetes.3
Disclosure
The authors report no conflicts of interest in this report.
References
2011 National Diabetes Fact Sheet. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available from: http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed April 7, 2014. | |
Gosmanov AR, Wall BM. Diabetic ketoacidosis. In: Bope ET, Kellerman RD, editors. Conn’s Current Therapy 2014. Philadelphia, PA: Elsevier Saunders; 2014:710–713. | |
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. | |
Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006;35(4):725–751, viii. | |
Randall L, Begovic J, Hudson M, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care. 2011;34(9):1891–1896. | |
Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350–357. | |
Gosmanov AR, Umpierrez GE, Karabell AH, Cuervo R, Thomason DB. Impaired expression and insulin-stimulated phosphorylation of Akt-2 in muscle of obese patients with atypical diabetes. Am J Physiol Endocrinol Metab. 2004;287(1):E8–E15. | |
Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(12):2739–2748. | |
Sheikh-Ali M, Karon BS, Basu A, et al. Can serum beta- hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. | |
Nyenwe EA, Kitabchi AE. Evidence-based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Res Clin Pract. 2011;94(3):340–351. | |
Caputo DG, Villarejo F, Valle GB, Díaz Aguiar P, Apezteguia CJ. [Hydration in diabetic ketoacidosis. What is the effect of the infusion rate?]. Medicina (B Aires). 1997;57(1):15–20. Spanish. | |
Basnet S, Venepalli PK, Andoh J, Verhulst S, Koirala J. Effect of normal saline and half normal saline on serum electrolytes during recovery phase of diabetic ketoacidosis. J Intensive Care Med. 2014;29(1):38–42. | |
Fisher JN, Shahshahani MN, Kitabchi AE. Diabetic ketoacidosis: low-dose insulin therapy by various routes. N Engl J Med. 1977;297(5):238–241. | |
Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93(5):1541–1552. | |
Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a priming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? Diabetes Care. 2008;31(11):2081–2085. | |
Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422–427. | |
Rosenthal NR, Barrett EJ. An assessment of insulin action in hyperosmolar hyperglycemic nonketotic diabetic patients. J Clin Endocrinol Metab. 1985;60(3):607–610. | |
Maletkovic J, Drexler A. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2013;42(4):677–695. | |
Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27(8):1873–1878. | |
Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117(5):291–296. | |
Ersöz HO, Ukinc K, Köse M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60(4):429–433. | |
Shen T, Braude S. Changes in serum phosphate during treatment of diabetic ketoacidosis: predictive significance of severity of acidosis on presentation. Int Med J. 2012;42(12):1347–1350. | |
Fisher JN, Kitabchi AE. A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab. 1983;57(1):177–180. | |
Winter RJ, Harris CJ, Phillips LS, Green OC. Diabetic ketoacidosis. Induction of hypocalcemia and hypomagnesemia by phosphate therapy. Am J Med. 1979;67(5):897–900. | |
Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18(10):2644–2648. | |
Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23(2):148–156. | |
Tzamaloukas AH, Ing TS, Siamopoulos KC, et al. Body fluid abnormalities in severe hyperglycemia in patients on chronic dialysis: review of published reports. J Diabetes Complications. 2008;22(1):29–37. | |
Tzamaloukas AH, Rohrscheib M, Ing TS, et al. Serum potassium and acid-base parameters in severe dialysis-associated hyperglycemia treated with insulin therapy. Int J Artif Organs. 2005;28(3):229–236. | |
Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2(1):162–174. | |
Warnock DG. Uremic acidosis. Kidney Int. 1988;34(2):278–287. | |
Gutierrez R, Oster JR, Schlessinger FB, Perez GO, Federman DG, Vaamonde CA. Serum sulfate concentration and the anion gap in hemodialysis patients. ASAIO Trans. 1991;37(2):92–96. | |
Axelrod L. Response of congestive heart failure to correction of hyperglycemia in the presence of diabetic nephropathy. N Engl J Med. 1975;293(24):1243–1245. | |
Tzamaloukas AH, Ing TS, Siamopoulos KC, et al. Pathophysiology and management of fluid and electrolyte disturbances in patients on chronic dialysis with severe hyperglycemia. Semin Dial. 2008;21(5):431–439. | |
Montoliu J, Revert L. Lethal hyperkalemia associated with severe hyperglycemia in diabetic patients with renal failure. Am J Kidney Dis. 1985;5(1):47–48. | |
Hsia E, Seggelke S, Gibbs J, et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab. 2012;97(9):3132–3137. | |
Savage MW, Dhatariya KK, Kilvert A, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28(5):508–515. | |
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Can J Diabetes. 2013;37 Suppl 1:S72–S76. | |
Bull SV, Douglas IS, Foster M, Albert RK. Mandatory protocol for treating adult patients with diabetic ketoacidosis decreases intensive care unit and hospital lengths of stay: results of a nonrandomized trial. Crit Care Med. 2007;35(1):41–46. | |
Waller SL, Delaney S, Strachan MW. Does an integrated care pathway enhance the management of diabetic ketoacidosis? Diabet Med. 2007;24(4):359–363. | |
Hara JS, Rahbar AJ, Jeffres MN, Izuora KE. Impact of a hyperglycemic crises protocol. Endocr Pract. 2013;19(6):953–962. | |
Devalia B. Adherance to protocol during the acute management of diabetic ketoacidosis: would specialist involvement lead to better outcomes? Int J Clin Pract. 2010;64(11):1580–1582. | |
Solá E, Garzón S, García-Torres S, Cubells P, Morillas C, Hernández-Mijares A. Management of diabetic ketoacidosis in a teaching hospital. Acta Diabetol. 2006;43(4):127–130. | |
Singh RK, Perros P, Frier BM. Hospital management of diabetic ketoacidosis: are clinical guidelines implemented effectively? Diabet Med. 1997;14(6):482–486. | |
Golden SH, Sapir T. Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review. J Manag Care Pharm. 2012;18(Suppl 6):S1–S17. | |
Peyrot M, Rubin RR. Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther. 2009;11(1):57–62. | |
Wallace TM, Matthews DR. Recent advances in the monitoring and management of diabetic ketoacidosis. QJM. 2004;97(12):773–780. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
