Back to Journals » International Medical Case Reports Journal » Volume 7
Magnetic resonance imaging features of brain and spinal cord injury in a fatal case of isopropanol intoxication
Authors Mahajan P, Mathew J, Jayaram A, Negi V, Abu Hmaira MM
Received 4 January 2014
Accepted for publication 29 January 2014
Published 24 March 2014 Volume 2014:7 Pages 57—61
DOI https://doi.org/10.2147/IMCRJ.S60082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Video abstract presented by Parag Suresh Mahajan
Views: 755
Parag Suresh Mahajan,1 Joyal Jacob Mathew,2 Abhilash Pulincherry Jayaram,1 Vidya Chander Negi,1 Mohamed Milad Abu Hmaira2
1Department of Radiology, 2Department of Medicine, Al-Khor Hospital, Hamad Medical Corporation, Doha, Qatar
Abstract: A 60-year-old man presented with headache, dizziness, and disorientation one day after consumption of isopropanol along with ethanol. Computed tomography (CT) of the brain performed immediately was unremarkable. The patient collapsed within the hospital 30 minutes after the CT scan was done, and remained comatose until death, showing no improvement with symptomatic treatment. Magnetic resonance imaging of the brain and spine done 6 days after admission revealed bilaterally symmetrical hyperintensities involving the cerebral and cerebellar cortex and white matter, basal ganglia, thalami, and brainstem on T2-weighted, fluid attenuated inversion recovery and diffusion weighted images; similar hyperintensities were seen involving the swollen and edematous cervical spinal cord and cerebellar tonsillar herniation compressing the proximal cervical cord. Petechial hemorrhages were also noted within the brainstem. These features are compatible with toxic injury to the brain and cervical spinal cord. To our knowledge, the magnetic resonance imaging features of brain and spinal cord injury and cerebellar tonsillar herniation, secondary to isopropanol intoxication have not been reported in the published literature before.
Keywords: alcohol intoxication, computed tomography, isopropyl alcohol, ethyl alcohol, toxicity
Introduction
The clinical effects of toxic alcohols are mainly due to depression of both the central nervous system and myocardial function.1 There are very few published reports of the magnetic resonance imaging (MRI) features of central nervous system involvement in various types of toxic alcohol poisoning.1–5 All reports mention cerebral and/or cerebellar toxicity, also known as toxic encephalopathy. There have been no published reports of brain and spinal cord injury secondary to isopropanol (isopropyl alcohol) intoxication.1–5
Case report
A 60-year-old Caucasian merchant ship captain presented to the emergency department of our hospital with headache, dizziness, and disorientation one day after ingestion of isopropanol along with ethanol (ethyl alcohol, drinking alcohol). He had celebrated his 60th birthday aboard ship by consuming ethanol and then isopropanol, because ethanol was not available later. His colleague accompanying him to the hospital brought an empty bottle of rubbing alcohol labelled as 70% volume per volume isopropyl alcohol with a capacity of 473 mL (one pint). The label on the bottle mentioned its indication for use as a first aid antiseptic and for rubbing and massaging. The patient’s colleague mentioned that the patient had consumed most of the liquid in the bottle and that he (the colleague) had consumed a small amount. The patient’s colleague did not give any recent or past history of substance abuse. The patient was disoriented and clinical examination was unremarkable. An unenhanced computed tomography (CT) scan of the brain was performed immediately and was unremarkable. He was admitted for further investigations and management. Symptomatic treatment was started.
The patient collapsed in the hospital just 30 minutes after the CT scan was done. He became deeply comatose with a score of 3/15 on the Glasgow Coma Scale (a scale to assess central nervous system status where a total score of 15 indicates best and normal outcome). He also developed hypotension, with a blood pressure of 80/40 mmHg, a pulse rate of 65 per minute, and a respiratory rate of 8 per minute. Generalized hypotonia and absent tendon reflexes were noted on neurological examination. There was no evidence of trauma to the head or cervical spine. After administration of intravenous fluids and endotracheal intubation, the patient was admitted to the intensive care department. Blood investigations revealed leukocytosis of 26,000 (normal 4,000–10,000) white blood cells per μL, a hemoglobin of 15.7 (normal 13–17) g/dL, and a mean corpuscular volume of 93 (normal 83–101) fl. The initial arterial blood gas report indicated a pH of 6.731 (normal 7.35–7.45), a pCO2 level of 28.2 (normal 35–48) mmHg, a pO2 level of 141 (normal 83–108) mmHg, a bicarbonate level of 3.5 (normal 21–28) mmol/L, and oxygen saturation of 95% (normal 95%–98%). Other blood investigations revealed a blood urea nitrogen of 9.5 (normal 1.7–8.3) mmol/L, serum creatinine of 157 (normal 62–124) μmol/L, Na+ of 141 (normal 134–146) mmol/L, K+ of 6 (normal 3.5–5) mmol/L, Cl− of 107 (normal 96–110) mmol/L, HCO3 of 5 (normal 24–30) mmol/L, and capillary blood glucose of 7.2 (normal 3.3–5.5) mmol/L. Investigations were suggestive of severe metabolic acidosis and acute renal failure. His blood lactic acid level was significantly elevated at 8.7 (normal 0.5–2.2) mmol/mL. Blood ketones were negative. Blood investigations were negative for the presence of ethanol. Blood levels of isopropanol and other toxic alcohols (like methanol, ethylene glycol, propylene glycol, and diethylene glycol) could not be obtained because the necessary investigations were not available. Liver enzymes, serum amylase, and lipase levels were normal.
Investigations repeated at 2-hour intervals revealed increasing renal impairment, hyperglycemia, and electrolyte imbalance (low bicarbonate levels and hyperkalemia). Follow-up arterial blood gas analysis again revealed severe acidosis. No growth was detected on cultures of urine and blood, and no crystals were found in the urine on microscopic examination. The serum pseudocholinesterase level was normal at 7,438 (normal 5,400–13,200) U/L. Calculated serum osmolarity was 310 (normal 280–301) mOsmol/L.
The patient received hemodialysis because of severe acidosis and hyperkalemia, which led to gradual improvement in his blood pH and lactic acid levels. He was also treated with norepinephrine (as a vasopressor) and intravenous fluids, but there was no improvement in blood pressure. He received 500 mL of fractionated plasma protein stat over 30 minutes, 2,000 mL of normal saline over 2 hours, and was then kept on 200 mL/hour of normal saline. He did not respond to intravenous fluids and within the first 2 hours was started on norepinephrine 10 μg per minute, which was gradually increased up to a maximum dose of 90 μg per minute. His blood pressure stabilized and he was weaned off norepinephrine after 4 days.
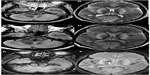
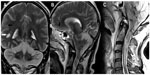
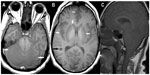
Unenhanced MRI scans of the brain and spine performed 6 days after hospital admission showed bilaterally symmetrical hyperintensities on T2-weighted, T1-weighted, T2*-weighted, fluid attenuated inversion recovery (FLAIR), and diffusion-weighted images in the cerebral and cerebellar cortex and white matter, basal ganglia, thalami, and brainstem (Figures 1–4). A swollen and edematous cervical spinal cord was noted with T2-weighted and FLAIR hyperintensities within it (Figures 2 and 4). Cerebellar tonsillar herniation of 17 mm was noted to be compressing the proximal cervical cord (Figures 2 and 4). Petechial hemorrhages were noted in the brainstem and the gangliocapsular regions bilaterally (Figure 1). All the features described above are compatible with toxic brain and cervical spinal cord damage. Finally, the patient expired ten days after hospital admission, despite his improving blood picture.
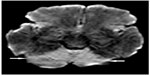  | Figure 3 Axial diffusion-weighted image showing restricted diffusion involving the entire cerebellum bilaterally (arrows). |
Discussion
Chemicals involved in alcohol intoxication are ethanol, methanol, isopropanol, ethylene glycol, diethylene glycol, and propylene glycol. Ethanol is considered as a drinking alcohol and the rest as toxic alcohols. Among the toxic alcohols, isopropanol itself is more toxic than its metabolite (acetone), while the metabolites of the rest of the toxic alcohols are more toxic than the parent alcohol. Isopropanol poisoning is characterized by an increased osmolal gap in the setting of positive serum and urine ketones and does not cause metabolic acidosis, while the rest of the toxic alcohols cause mild to severe metabolic acidosis.1 When not mixed with ethanol or other intoxicants, the signs and symptoms of intoxication may start earlier (few hours) after isopropanol ingestion and may be delayed by up to a day or more after ingestion of other toxic alcohols. Unlike methanol and ethylene glycol, isopropanol is more toxic than its metabolites; hence, alcohol dehydrogenase inhibitors are not given.1 Hemodialysis removes both isopropanol and its metabolite.1 In our case, the clinical and biochemical features were atypical for isopropanol toxicity likely due to coconsumption of ethanol and delayed patient presentation. Ingestion of approximately 200 mL of pure isopropanol can be lethal.1 The patient was managed symptomatically, mainly with hemodialysis. We wish to emphasize the atypical MRI features in this case that have not been reported before in cases of toxic alcohol ingestion.
In our case, MRI revealed ischemic lesions involving the brain and cervical spinal cord. These were likely due to reduced brain perfusion.6 These lesions were bilaterally symmetrical, and the distribution of the affected areas was highly indicative of a toxic injury.6 Bilateral involvement of the basal ganglia and thalami has been reported in a few cases of ethylene glycol intoxication. However, cervical spinal cord involvement and cerebellar tonsillar herniation has not been reported before in cases of toxic alcohol ingestion and even in cases of other substance abuse.
In humans, the opioid receptors are predominantly present in the cerebellum and the limbic systems, thus causing cerebellar-predominant toxicity in cases of opioid intoxication.6 Involvement of the basal ganglia is also noticed in a few of these cases.6 A similar explanation may be given in cases of alcohol intoxication. To the best of our knowledge, this is the first reported case of cerebral, cerebellar, brainstem, and cervical spinal cord involvement on MRI secondary to isopropanol intoxication.
Conclusion
Here we present a very rare case of involvement of cerebrum, cerebellum, brainstem, and cervical spinal cord demonstrated on MRI after coconsumption of isopropanol and ethanol. To our knowledge, involvement of the cervical spinal cord and cerebellar tonsillar herniation after toxic alcohol ingestion have not been reported before in the published literature.
Disclosure
The authors report no conflicts of interest in this work.
References
Emadi A, Coberly L. Intoxication of a hospitalized patient with an isopropanol-based hand sanitizer. N Engl J Med. 2007;356(5):530–531. | |
Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol. 2008;3(1):208–225. | |
Jammalamadaka D, Raissi S. Ethylene glycol, methanol and isopropyl alcohol intoxication. Am J Med Sci. 2010;339(3):276–281. | |
Sharma P, Eesa M, Scott JN. Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol. 2009;193(3):879–886. | |
Agarwal A, Vancil T. Toxic encephalopathy. J Gen Intern Med. 2012;27(7):876–877. | |
Corré J, Pillot J, Hilbert G. Methadone-induced toxic brain damage. Case Rep Radiol. 2013;2013:602981. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.