Back to Journals » Patient Preference and Adherence » Volume 8
Lung vital capacity and oxygen saturation in adults with cerebral palsy
Authors Lampe R, Blumenstein T, Turova V , Alves-Pinto A
Received 11 August 2014
Accepted for publication 29 September 2014
Published 9 December 2014 Volume 2014:8 Pages 1691—1697
DOI https://doi.org/10.2147/PPA.S72575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Renée Lampe,1,2 Tobias Blumenstein,2 Varvara Turova,2 Ana Alves-Pinto2
1Markus Würth Stiftungsprofessur, Technical University of Munich, Munich, Germany; 2Research Unit for Cerebral Palsy and Children Neuroorthopaedics of the Buhl-Strohmaier Foundation, Orthopedic Department of the Clinic “rechts der Isar” of the Technical University of Munich, Munich, Germany
Background: Individuals with infantile cerebral palsy have multiple disabilities. The most conspicuous syndrome being investigated from many aspects is motor movement disorder with a spastic gait pattern. The lung function of adults with spasticity attracts less attention in the literature. This is surprising because decreased thoracic mobility and longstanding scoliosis should have an impact on lung function. With increasing age and the level of disability, individuals become susceptible to lung infections and reflux illness, and these are accompanied by increased aspiration risk. This study examined, with different methods, to what extent adults with congenital cerebral palsy and acquired spastic paresis – following traumatic brain injury – showed restriction of lung function. It also assessed the contribution of disability level on this restriction.
Methods: The oxygen saturation of 46 adults with a diagnosis of cerebral palsy was measured with an oximeter. Lung vital capacity was measured with a mobile spirometer and excursion of the thorax was clinically registered. The gross motor function levels and the presence or absence of scoliosis were determined.
Results: A significantly positive correlation between lung vital capacity and chest expansion was established. Both the lung vital capacity and the thorax excursion decreased with increases in gross motor function level. Oxygen saturation remained within the normal range in all persons, in spite of reduced values of the measured lung parameters. No statistically significant dependency between lung vital capacity and oxygen saturation, and between chest expansion and oxygen saturation was found. The scoliotic deformities of the spine were associated with an additional decrease in the vital capacity, but this did not affect blood oxygen supply.
Conclusion: Despite the decreased chest expansion and the significantly reduced lung volume in adults with cerebral palsy, sufficient oxygen supply was registered.
Keywords: disability, lung function, spirometry, chest expansion, pulse oximetry
Introduction
Cerebral palsy (CP) affects about 2–3 per 1,000 newborns, and it is one of the most frequent reasons for disability in the world.1 Besides neurological movement disorders, CP comprises different syndromes and problems including respiratory problems, which especially affect people with severe disabilities. A typical problem noted in premature infants is bronchopulmonal dysplasia, which causes pneumonia and respiratory failure, as well as influenza or infection of the lower respiratory tract.2 In a study of respiratory function of spastic children, the lung vital capacity (VC) was measured with a spirometer and compared with that of able-bodied persons.3 Spastic diplegic children showed the most reduced VC, and their respiratory function was more affected than in hemiplegic children. Also, during the feeding of persons with severe disabilities who also suffer from gastrointestinal reflux, aspiration could happen and lead to aspiration pneumonia.4 The oxygen saturation (OS) level, as measured by pulse oximetry, decreased during feeding in the sitting position because of the stress on the circulatory system.5 Reid et al studied the causes of death among persons with CP for a period of 35 years.2 The most common direct causes of death were respiratory-related. To improve the pulmonary function, early respiratory exercises are important and have to be included in the therapy programs for people with CP.6
Not only lung function, but also expansion (excursion) of the chest, can influence respiratory function. Decreased chest expansion (CE), as measured by chest circumference in maximal inspiration and expiration, was detected in children with spastic CP.7 CE was significantly decreased when compared to healthy people, and it also decreased with increasing age.
In our study, OS, VC, and CE were measured in adults with CP. The dependency of VC on CE and on Gross Motor Function Classification System (GMFCS) mobility level was also examined. The GMFCS is an internationally recognized classification system that assesses motor function and assigns an individual one of five levels, with higher levels indicating more severe motor impairment. Additionally, we investigated the influence of clinically diagnosed scoliosis on VC, CE, and OS. Our aim was to gain a better understanding of the main factors associated with breathing problems in people with CP by cross-comparing information about lung and respiratory function, motor impairment, and other physical parameters.
Methods
Sample
Forty-six adults aged from 22–59 years diagnosed with CP were examined. Most of them had congenital CP; only four had the same syndromes (ie, tetraparesis acquired after traumatic brain injury many years ago). Four patients (8.7%) were classified as GMFCS level I, three (6.5%) as GMFCS level II, 18 (39.1%) as GMFCS level III, and 21 (45.7%) as GMFCS level IV. The presence or absence of scoliosis was determined by an experienced orthopedic surgeon. Sixteen of the patients (34.8%) were diagnosed as having scoliosis. All individuals were involved in the general rehabilitation and training program at a center for persons with CP. The therapy program ran during the patients’ work day and was comprised of speech therapy, physiotherapy, and occupational therapy, as well as respiratory exercises. To protect each participant’s identity, no personal information (eg, age, weight, diagnosis, height, sex) is disclosed here. All experimental procedures were approved by the ethics committee of the faculty of medicine of the Technical University of Munich (reference 43/14). Informed consent was obtained from all participants before starting the measurements.
Spirometry
VC was measured with a spirometer (MiniSpir®) from MIR (Medical International Research Srl, Rome, Italy). The spirometer was connected to a computer via a universal serial bus (USB) cable and controlled via the software supplied. For hygiene reasons, individual disposable turbines were used to perform the measurements.
During the measurement, the participant was asked to completely enclose the turbine with his or her mouth, to inhale up to his or her maximum capacity, and then to exhale with the maximum force possible. The VC was determined as the difference in the volume between maximal inhalation and maximum exhalation. The measurement was repeated four to five times, and the maximum value was chosen. Some persons could not enclose the turbine completely with their mouths. In these cases, a mouthpiece made of rubber was used to help stream the inhaled air into the turbine.
Oxygen saturation (pulse oximeter)
OS refers to the concentration of oxygen in the blood. The values in the range of 96%–100% are considered normal. To measure the heart rate and OS in the blood, a pulse oximeter (PO80; Beurer GmbH, Ulm, Germany) was used. The pulse oximeter is ideal for measurements among people with CP because it is noninvasive, fast, and has no disturbing effects.
During the measurement, the oximeter was simply clamped to the tip of a finger and the result was read directly from the device. Alternatively, the oximeter was connected to a computer via a USB to record the course of OS and heart rate. Recordings were made over a period of 5 minutes with a sampling rate of 1 Hz. In the evaluation, an average value over the entire measurement period was calculated.
Chest expansion
CE was measured with a tapeline for maximum inspiration and expiration, and also during normal breathing. The tapeline was positioned slightly below the mammillary line. CE was calculated as the difference, in cm, between the perimeter of the chest at maximum inspiration and that at full expiration. To compare the measured values of CE with the normal values reported by Moll and Wright,8 the patients were assigned to different groups based on age.
Statistical analysis
The data were analyzed using the R-package STATS for Windows.9 We investigated the relationship between VC and CE. The association between OS and CE and the relationship of OS to the deviation of the measured CE (DCE) from the norm were analyzed. We also examined the association between OS and VC and between OS and the deviation of VC (DVC) from the norm. The norm values of VC were calculated using the following formulas:10
VC (male, in l) = 10−3 × (27.63 – (0.112 × age)) × height (cm) | (1) | |
VC (female, in l) = 10−3 × (21.78 – (0.101 × age)) × height (cm) | (2) |
A comparison of the DCE, DVC, and OS between patients with and without scoliosis was carried out. In the statistical analysis, Student’s t-test, analysis of variance (ANOVA), Pearson’s correlation, and the χ2 test were used, depending on the variables and objectives. The significance was set at P<0.05.
Results
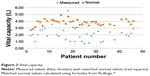
For all individuals, independent of the severity of the diagnosis and GMFCS, the OS showed physiological values (Table 1). The mean values of CE were below the normal values in all age groups8 (see Table 1 and Figure 1). For example, in the age group of 25–34 years, the mean CE was 4.37 cm smaller for males and 3.21 cm smaller in females when compared to the normal values. The values of VC were also lower than the matched norm values (see Table 1 and Figure 2).
 
|
Table 1 Measurement results |
 
|
Figure 1 Chest expansion. |
|
Figure 2 Vital capacity. |
Both CE and VC varied inversely with the GMFCS: the greater the level of severity, the lower the mean values of CE and VC that were measured (see Figures 3 and 4). This dependency was not statistically significant for CE (ANOVA, P=0.08), but it was significant for VC (ANOVA, P=0.012). Despite the decrease in CE and VC with GMFCS, no changes in OS with GMFCS level were observed (ANOVA, P<0.12).
We verified the hypothesis that VC and CE were correlated, and the degree of their functional dependency. To this end, polynomial predictors of degree n, n=1–6 based on the nonlinear regression analyses that were used.9 The computed R2 values (the coefficients of determination) and Pearson’s coefficients (r) of the correlation between the measured and predicted values are presented in Table 2. Both R2 values (the coefficients of determination) and Pearson’s coefficients (r) of correlation are sufficiently large. Additionally, Pearson’s correlation test confirms the significant correlation between the measured and predicted values, with P-values <0.05 beginning from n=2.
The hypothesis on the presence of a correlation between CE and OS, as well as their degree of functional dependency, was verified. As expected, the R2 values grow with n, but they remain sufficiently small (see Table 2), which points to the absence of significant functional dependency between OS and CE. Pearson’s correlation coefficients are also sufficiently small. Moreover, Pearson’s correlation test proves the hypothesis that there is zero correlation between the measured and predicted values, with relative large P-values (Table 2). Additionally, the nonparametric χ2 test shows a high level of independence of the measured and predicted random values for all n. The magnitude of P-values for the χ2 test is about 0.35 for all n. These results show the low likelihood of the presence of a functional dependency between OS and CE.
The analogous statistical results listed in Table 2 show the absence of the significant correlation and functional dependency between OS and the DCE from the norm values reported by Moll and Wright;8 see the computed R2 values, Pearson’s correlation coefficients, and P-values. Moreover, a significant correlation and functional dependency between OS and VC and between OS and the DVC was also absent (see the corresponding R2 values, Pearson’s correlation coefficients, and P-values listed in Table 2).
To study the influence of scoliosis on VC and CE, two groups of individuals were considered: the first one included 15 people with scoliosis (SG); and the second one contained 23 people without scoliosis (NSG). We examined the DVC from the norm values computed by Equations 1 and 2. The mean value of the DVC for the SG was equal to 2.189±0.434 L, the mean value for the NSG was equal to 1.216±0.388 L. This difference is statistically significant (t-test, P<0.001); that is, the two groups have different DVCs.
The second question concerned the deviation, DCE, in the SG and NSG. The mean values were equal to 4.006 cm for SG and 3.382 cm for NSG. The difference was not statistically significant (t-test, P=0.365). Nevertheless, it should be noted that the 95% confidence interval for the t-test (-0.763 to 2.013) shifted towards positive values, which indicates that the DCE for the SG is greater than that for the NSG; that is, the SG showed a larger deviation from the normal values than did the NSG.
It should be noted that no significant difference in the OS for the SG (mean ± standard deviation [SD]=96.33%±1.35%) and the NSG (mean ± SD=97.086%±0.521%) was found (t-test, P=0.668). The confidence interval (-2.175 to 0.668) shifted towards negative values, indicating that – though not significantly different – the mean OS for the SG is lower than that for the NSG. Nevertheless, both means fell in the range of normal values.
Discussion
Similar to Ezeugwu, who compared lung function in people with stroke and healthy controls,12 the chest excursion in our study was reduced in all age groups both for male and female individuals, as compared to the norm values reported by Moll and Wright.8 The reduced CE associated with CP is caused by weakness of the trunk muscles and difficulty in stabilizing trunk posture. However, the decreased CE does not affect the level of blood OS, which remains in the range of normal values.
Hutzler et al13 observed reduced VC in children with CP when compared to matched healthy persons. Improvement of lung function was achieved via a swimming program that took place two times a week for 6 months. In our study, VC was also decreased when compared to the calculated aged-, body height- and sex-matched normal values. Nevertheless, the decreased VC did not influence OS in adults with CP.
According to our findings, scoliotic deformity of the spine causes additional restriction of the functionally related VC and CE, which is in agreement with the results by Campbell et al.14 Kalen et al15 compared OS in two groups of individuals with quadriplegia: one group had severe scoliosis; another group had mild or no curves. No differences in the OS levels were registered. In our study, no difference was established between the measured mean OS values for persons with scoliosis and those without scoliosis, which suggests that oxygen supply in adults can reach normal levels despite the larger DVC from normality with a diagnosis of scoliosis.
The OS values were at physiological levels, despite the reduced VC and CE, the lack of correlation between VC and OS, and the lack of correlation between CE and OS. This suggests that there is an adaptation in the bodies of persons with CP to their reduced mobility, which is reflected in the normal OS values. The natural question that arises is how does OS remain at normal levels? All patients in this study underwent physiotherapy and performed respiratory exercises several times a week. Also, they frequently had different therapies like Bobath physiotherapy, breathing exercises, and speech and swallowing therapies to prevent contractures and to retain their mobility and facilitate their breathing. Moreover, they were regularly examined by an experienced orthopedic surgeon and internal doctor. Considering the positive results that swimming exercises have been observed to have on the enhancement of VC in children with CP,13 the normal levels of OS observed here likely reflect the training that these patients receive every week. Physical therapy and training programs should therefore be a part of schools and centers for persons with physical disabilities. Moreover, the mobility of individuals with CP is reduced, which suggests that they do not need the same physiological VC and CE as normally developed, healthy adults.
Conclusion
A significant functional relationship between lung VC and the CE of adults with CP was established. Despite the reduced VC and decreased CE, with the exception of four patients that presented with levels between 90% and 94%, the blood OS levels were not lower than 95% for the majority of patients involved in the study. No significant functional dependencies were found between VC and OS and between CE and OS. Even in patients with scoliotic deformities of the spine, which contributed to a reduction in lung VC and CE, oxygen supply within the normal range was observed. We concluded that the body systems of adults with CP involved in regular physical therapy and training programs adapt to individual conditions over the long term to retain sufficient oxygen supply.
Acknowledgments
The authors thank Stiftung Würth and Buhl-Strohmaier Stiftung for their financial support. The authors are also grateful to Dr Nikolai Botkin, Center of Mathematics, Technical University of Munich, for his help with statistical analysis of the data.
Disclosure
The authors report no conflicts of interest in this work.
References
Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disability and Rehabilitation. 2006;28(4):183–191. | ||
Reid SM, Carlin JB, Reddihough DS. Survival of individuals with cerebral palsy born in Victoria, Australia, between 1970 and 2004. Dev Med Child Neurol. 2012;54(4):353–360. | ||
Kwon YH, Lee HY. Differences of respiratory function in children with spastic diplegic and hemiplegic cerebral palsy, compared with normally developed children. J Pediatr Rehabil Med. 2013;6(2):113–117. | ||
Satou Y, Oguro H, Murakami Y, et al. Gastroesophageal reflux during enteral feeding in stroke patients: a 24-hour esophageal pH-monitoring study. J Stroke Cerebrovasc Dis. 2013;22(3):185–189. | ||
Tamura F, Shishikura J, Mukai Y, Kaneko Y. Arterial oxygen saturation in severely disabled people: effect of oral feeding in the sitting position. Dysphagia. 1999;14(4):204–211. | ||
Rothman JG. Effects of respiratory exercises on the vital capacity and forced expiratory volume in children with cerebral palsy. Phys Ther. 1978;58(4):421–425. | ||
Ersöz M, Selçuk B, Gündüz R, Kurtaran A, Akyüz M. Decreased chest mobility in children with spastic cerebral palsy. Turk J Pediatr. 2006;48(4):344–350. | ||
Moll JM, Wright V. An objective clinical study of chest expansion. Ann Rheum Dis. 1972;31(1):1–8. | ||
Marques de Sá JP. Directional data. In: Applied Statistics Using SPSS, STATISTICA, MATLAB and R. 2nd ed. Berlin Heidelberg:Springer. | ||
Rollings RC. Facts and Formulas. 4th ed. Saline, MI: McNaughton & Gunn; 1985. | ||
Wang T, Zhang S. Study on linear correlation coefficient and nonlinear correlation coefficient in mathematical statistics. Studies in Mathematical Sciences. 2011;3(1):58–63. | ||
Ezeugwu VE, Olaogun M, Mbada CE, Adedoyin R. Comparative lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother Res Int. 2013;18(4):212–219. | ||
Hutzler Y, Chacham A, Bergman U, Szeinberg A. Effects of a movement and swimming program on vital capacity and water orientation skills of children with cerebral palsy. Dev Med Child Neurol. 1998;40(3):176–181. | ||
Campbell RM Jr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85-A(3):399–408. | ||
Kalen V, Conklin MM, Sherman FC. Untreated scoliosis in severe cerebral palsy. J Pediatr Orthop. 1992;12(3):337–340. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



