Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies
Authors Gomes MJ, Martins S, Ferreira D, Segundo M, Reis S
Received 22 March 2013
Accepted for publication 24 May 2013
Published 7 March 2014 Volume 2014:9(1) Pages 1231—1242
DOI https://doi.org/10.2147/IJN.S45561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Maria João Gomes,1 Susana Martins,2,3 Domingos Ferreira,2 Marcela A Segundo,1 Salette Reis1
1REQUIMTE, Laboratory of Applied Chemistry, Department of Chemical Sciences, Faculty of Pharmacy, 2Laboratory of Pharmaceutical Technology, Department of Drug Sciences, Faculty of Pharmacy, University of Porto, Porto, Portugal; 3Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, Odense, Denmark
Abstract: Alopecia is a dermatological disorder, commonly known as hair loss, which affects up to half of the Caucasian male population by middle age, and almost all (95%) Caucasian men by old age. Considering that alopecia affects so many people and that there is currently no scientifically proven treatment with few side effects, new drug-delivery systems able to improve alopecia therapy are urgently required. With this purpose in mind, the present study aimed to develop lipid nanoparticles (nanostructured lipid carriers) with the ability to incorporate and deliver anti-alopecia active compounds (minoxidil and finasteride) into the dermis and hair follicles. Lipid nanoparticles, prepared by ultrasonication method, showed mean particle sizes around 200 nm, which is sufficient for reaching the dermis and hair follicles, and zeta potential values around -30 mV, which indicates good physical stability. Over 28 days of storage, no significant variations in these parameters were observed, which indicates that all nanoformulations are stable in storage over that period. Cryo-scanning electron microscope measurements showed that all the lipid nanoparticles exhibited a spherical shape and a smooth surface regardless of their composition. Differential scanning calorimetry studies allowed the determination of phase transition temperatures and confirmed the recrystallization of the lipid nanoparticles (recrystallization index between 11% and 86%). A high loading efficiency was achieved for finasteride (between 70% and 90%), while less than 30% was achieved for minoxidil nanoparticles, over 28 days. Controlled release assays in physiological conditions demonstrated that nanoparticles loaded with minoxidil yielded a prolonged release, as desired. Penetration assays through pig ear skin demonstrated that nanoparticles loaded with minoxidil and finasteride had low levels of penetration. These results suggest that the proposed novel formulation presents several good characteristics indicating their suitability for dermal delivery of anti-alopecia active compounds.
Keywords: nanostructured lipid carriers (NLC), alopecia, anti-alopecia therapy, minoxidil, finasteride
Introduction
Alopecia, commonly known as hair loss, results from a reduction of visible hair. This disease has different possible forms; androgenic alopecia (AGA) is a common form of hair loss in both men and women.1 It affects up to half of the Caucasian male population by middle age, and almost all (95%) Caucasian men by old age (80 years).2 This type of alopecia is progressive and related to a perturbation of the hair follicle cycle, which is caused by a shortening of the anagen stage, with the clinical consequence of increased hair loss accompanied by a transformation of terminal hair into cosmetically unsatisfactory vellus hair.3 The name AGA reflects the importance of both androgens (especially dihydrotestosterone [DHT]) and genes in this disorder etiology. Alopecia areata is another form of alopecia, which affects almost 0.1% of the general worldwide population and is characterized as a chronic, inflammatory disease resulting in an unpredictable, nonscarring form of hair loss.4,5 Considering that alopecia affects so many people and that no scientifically proven treatment for alopecia, with few side effects, is currently available, the development of new drug-delivery systems able to improve alopecia therapy is urgently required.
Lipid nanoparticles (LNs), such as solid LNs and nanostructured lipid carriers (NLCs), are composed of physiological (biocompatible) and biodegradable lipids,1 which possess low cytotoxicity and low systemic toxicity and thus high tolerability.6–8 LNs exhibit many advantageous features for dermal application of cosmetic and pharmaceutical products, such as: controlled drug release and drug targeting; improved drug physical stability; feasibility to incorporate lipophilic and hydrophilic drugs; avoidance of organic solvents; and no problems with respect to large-scale production and sterilization.9,10 Furthermore, LNs have occlusive properties that can increase the water content of the skin and that favor drug penetration into the skin,7,8 and readily interact with skin lipids.11 LNs also protect the loaded compounds against chemical degradation.12 Given these advantageous features, this type of nanocarrier can be used for the incorporation of anti-alopecia drugs.
From all the drugs used for alopecia therapy reported in the literature, minoxidil and finasteride were chosen to be incorporated into LNs in this study. Finasteride, an antiandrogen, is a synthetic steroid that selectively inhibits the action of the type II 5α-reductase enzyme.2,13 This enzyme converts testosterone to DHT, which is an androgen involved in hair growth. Low levels of DHT/inhibition of 5α-reductase is related to low levels of alopecia. The cessation of treatment recommences the balding process, indicating that the effects of finasteride are not curative.2 On the other hand, minoxidil is a hydrophilic compound widely used to treat alopecia, but its mechanism of action is not so well known. It is a vasodilator initially approved as a drug to control hypertension, by which it was discovered that it could aid in hair growth. It is known that, as is also the case with finasteride, minoxidil does not permanently inhibit hair loss processes: cessation of minoxidil treatment is followed by rapid shedding of hair, returning the scalp to its untreated state.2 The effects of both drugs could be synergic and this could be an advantage of this formulation.
The present work aims to explore the nanotechnology potential in alopecia therapy, based on the development and optimization of lipid nanocarriers (NLCs) that were characterized and evaluated for their stability. The suitability of these NLCs as carriers for dermal and transdermal delivery of anti-alopecia drugs was also assessed by the evaluation of several important parameters, such as particle size; zeta potential; degree of crystallinity, entrapment efficiency of the drugs into the carriers; their in vitro release, assessed by dialysis bag diffusion technique; and in vitro penetration through pig ear skin. Given the ease of application and the possible synergic effect obtained by the combination of two pharmaceutical agents, the formulation should constitute a great alternative to current alopecia treatments. The novelty of this work is the possible synergic effect, the systematic optimization, and the combination of several analyses (ie, the rigorous stability control, in vitro release, and penetration studies).
Materials and methods
Materials
Cetyl palmitate and Precirol® ATO 5 were kindly provided by Gattefossé (Lyon, France); oleic acid was purchased from Sanofi-Aventis (London, UK), and Miglyol® 812 was purchased from Acofarma (Madrid, Spain). Polysorbate 60 (Tween 60) was obtained from Merck KGaA (Darmstadt, Germany). All chemicals and solvents were of analytical grade and were used without further purification. Aqueous solutions were prepared with double-deionized Milli-Q water (Arium Pro, Sartorius AG, Göttingen, Germany). (conductivity less than 0.1 μS cm−1). Minoxidil and finasteride were purchased from Sigma-Aldrich (St Louis, MO, USA).
Methods
Preparation of NLCs
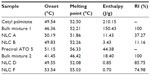
The NLCs were produced by ultrasonication method.14 Briefly, the excipients (lipids and surfactant) and the drug were mixed together and kept at temperature above the lipids’ melting point (70°C) to promote their mixture. The melted lipid phase (containing both solid and liquid lipids) was dispersed in a hot aqueous phase (containing the stabilizer polysorbate 60) to obtain a microemulsion by high-speed stirring using a T 25 ULTRA-TURRAX® (IKA®-Werke GmbH and Co, KG, Staufen, Germany) at 7,000 rpm for 60 seconds. This microemulsion was homogenized with sonication (Vibra-Cell™; Sonics and Materials, Inc, Newtown, CT, USA). The sonicator regulable frequency used was 70%, for 10 minutes to obtain a nanoemulsion, which was cooled at room temperature, allowing the inner oil phase to solidify and forming NLCs dispersed in an aqueous phase. The nanoparticles obtained were passed through a 200 nm filter (MiniSart® NML syringe filters; Sartorius AG, Göttingen, Germany) to remove aggregates and were transferred to 20 mL aluminum-sealed screw-neck vials (La-Pha-Pack® GmbH, Langerwehe, Germany) for sample storage purposes before posterior analyses. To understand the drug effect in the LNs and to determine the best concentration of the anti-alopecia drugs, different drug concentrations were encapsulated in the LNs based on the literature (2% and 3% of the total volume of lipids and surfactant for minoxidil;15 0.8% and 2% of the total volume of lipids and surfactant for finasteride16). Three batches of each formulation were developed to assess the reproducibility. The choice of the best percentage of each drug to use was based on the analyses of the size, zeta potential, and loading efficiency values of the nanoparticles produced. The composition of the formulations for each drug is shown in Table 1.
  | Table 1 Composition of the developed NLC formulations (mg) |
NLC characterization: size, surface charge, morphology, lipid polymorphism, and loading efficiency
The particle size and zeta potential of each batch produced were assessed using a BI-MAS multi-angle particle sizing device and ZetaPALS zeta potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). Analyses of the particle size were performed by dynamic light scattering (DLS), which yields the mean particle size and the polydispersity index (PI) as a measure of the width of the size distribution. Zeta potential is the electric potential of a particle in a suspension and was determined by electrophoretic light scattering. All the formulations were diluted 400-fold in water and tested at 25°C, both for size and zeta potential measurements. For size analysis, the real refractive index and the imaginary refractive index were set at 1.590 and 0, respectively. For each sample, the mean diameter ± standard deviation of six determinations was calculated applying multimodal analysis. For zeta potential analyses, an electric field was applied and surface-charged particles within the dispersion migrated toward the electrode of opposite charge, which is related to zeta potential values. The zeta potential results reported are the mean ± standard deviation of six runs of six cycles each from one sample. The mean particle size and PI and zeta potential values obtained were calculated from the three different batches for each concentration of drug tested.
Morphological evaluation (shape and size) of the nanoparticles prepared was performed using scanning electron microscopy (SEM). Measurements in this work used the cryo-SEM technique and were prepared by dropping the dispersions of the nanoparticles on copper grid-supported formvar films. The samples were frozen in liquid N2, cryofractures were made using an ALTO 2500 (Gatan, Pleasanton, CA, USA), and sublimation occurred. Samples were then observed using a JSM 6301F microscope (−150°C; JEOL, Tokyo, Japan), and analyzed with INCA Electronic Data System (EDS). EDS used in conjunction with the SEM apparatus is INCA Energy 350 (Oxford Instruments, Witney, UK) at the Materials Centre of the University of Porto, Porto, Portugal (CEMUP).
Differential scanning calorimetry (DSC) measurements were performed to obtain information about the melting behavior, polymorphism, and degree of crystallinity of the LNs under investigation and of the bulk materials used in the preparation of the nanoparticles. DSC measurements were carried out using a Pyris 1 differential scanning calorimeter (PerkinElmer, Waltham, MA, USA), and data were analyzed with the included software. The samples were weighed (2–11 mg) directly in aluminum pans and scanned between 30°C and 60°C, at a heating rate of 5°C/minute and cooling rate of 40°C/minute, under nitrogen gas. An empty aluminum pan was used as reference. These measurements were performed for three determinations. The degree of crystallinity or recrystallization index (RI) was determined by the following equation:
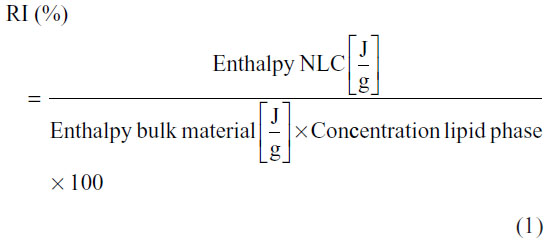
To imitate the production conditions, the solid lipids (cetyl palmitate and Precirol ATO 5) and bulk mixtures were heated a second time in the DSC and the values obtained were compared with those obtained for the NLCs (which were heated only once in the DSC).
Loading efficiency is another important parameter by which to characterize nanoparticles. This should be high enough to avoid losing drugs during the production and also to avoid the need of using a higher concentration of drugs. To determine loading efficiency, the nanoformulations were centrifuged (Jouan BR4i multifunction centrifuge, Thermo Electron Corporation, Madrid, Spain) through centrifugal filter units (Amicon Ultra-4, PLGC Ultracel-PL Membrane, 10 kDa; EMD Millipore, Billerica, MA, USA) at 4,000 rpm (3,300 × g) at 25°C for 5 to 15 minutes or until complete separation between the nanoparticles (pellet retained in the filter unit) and the aqueous phase (supernatant). The supernatant containing the dissolved free drug was analyzed in a PerkinElmer Lambda 45 UV/VIS spectrometer. Spectra were taken in the range of 200–800 nm for both drugs. Spectra of the nanoparticles without the drug incorporated (placebo) were also analyzed to establish the absorbance baseline. By considering the total amount of drug initially added to the formulation and subtracting the free drug that remained in the supernatant (ie, drug that was not incorporated in the NLCs), it is possible to obtain the amount of drug that was incorporated in the NLCs and thus determine the drug loading efficiency by the following equation:

Evaluation of NLC stability – control of size, zeta potential, and loading efficiency
Stability tests are important for providing evidence on how the quality of a formulation changes with time under the influence of a variety of conditions. As such, some parameters (size, zeta potential, and loading efficiency) were measured over 28 days of investigation to assess the stability of the formulations. Determination of particle size allows for verification of particle’s time-dependent stability regarding the tendency for aggregation and sedimentation. The particle charge determination is also important for predicting the stability of the formulation, since highly charged nanoparticles will be more stable, given that electric repulsion prevents nanoparticle aggregation.17,18
The NLC characterization (particle size and zeta potential) was done on the day of production and 7, 18, and 28 days later. The particle size and zeta potential were assessed by DLS and electrophoretic light scattering, respectively, as previously described.
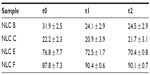
In order to evaluate NLC stability, loading efficiency was also measured over 28 days, in all three batches, on the day of production and 13 and 28 days after, to analyze possible drug expulsion from the LNs during storage. The methodology was as previously described.
In vitro controlled release studies
In vitro release of anti-alopecia drugs (minoxidil and finasteride) from LNs was evaluated using a dialysis bag diffusion technique. The controlled release studies were performed at physiological conditions corresponding to pH and temperature of the plasma (7.4 and 37°C, respectively) to evaluate the drug release profile at the conditions encountered by the formulation after dermal application. The drug-loaded NLC suspensions (1 mL for finasteride; 2.5 mL for minoxidil) were placed in cellulose dialysis bags with a 3,500 Da molecular weight cut-off (Cellu•Sep T1; Frilabo, Maia, Portugal), which were sealed at both ends. The dialysis bag was immersed in the receptor compartment containing 80 mL (in minoxidil experiments) or 600 mL (in finasteride experiments) phosphate buffer (pH 7.4 at 37°C), which was stirred at 100 rpm over 24 hours in a water bath placed over a stirring plate (Multistirrer magnetic stirrer, Velp Scientifica, Milan, Italy). Aliquots were removed from 1 mL dissolution medium to eppendorfs at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 9, 23, and 24 hours after the beginning of the experiment, and the same volume of fresh buffer was added periodically. This study was carried out under sink conditions: drug concentration in the medium was kept, at the very least, three times lower than the saturation solubility of the drugs in phosphate buffer. The samples were analyzed using a UV spectrophotometer (λ = 200–400 nm, to cover the absorbance maximum of each drug).
In vitro penetration studies
Penetration studies are important for assessing a formulation’s ability to go through a barrier – in this case, skin – which is crucial to their achieving their desired function. With these studies, therefore, it is possible to establish a drug formulation’s penetration profile.
Initially, pig ear skin was excised and dissected. Skin surface was cleaned and hairs were removed. With a scalpel and forceps, skin was excised. Then, skin was divided into pieces to be placed in Franz diffusion cells, and subcutaneous fatty tissue was removed. The pieces were frozen (−20°C) in plastic bags for storage. Franz diffusion cell experiments were made in triplicate for all formulations.
The receptor compartment was filled with phosphate buffer at pH 7.4, maintained at 37°C, and stirred constantly during all experiments. The skin was positioned between the two compartments of Franz cells and, in the donor compartment, 1 mL of NLC was placed. Samples were taken at predetermined time intervals (30 minutes and 1, 1.5, 3, 6, 9, 10, 22, 23, and 24 hours) from the sampling port; the same volume removed was replaced with buffer. Care was taken to avoid the formation of air bubbles between the skin and the acceptor buffer.
The determination of the drug content in the acceptor aliquots was done using a PerkinElmer Lambda 45 UV/VIS spectrometer for minoxidil, and high-performance liquid chromatography (HPLC) for finasteride. Chromatographic determination was performed in a liquid chromatography device using a MD-2015 multi-wavelength detector (Jasco, Easton, MD, USA) programmed for peak detection at 210 nm, a high-pressure pump (PU-2080), and a controller (LC-Net II/ADC, Jasco) controlled by ChromNAV software, Jasco. A reversed-phase monolithic column (Chromolith RP-18e, 100 × 4.6 mm; Merck) connected to a guard column of the same material (5 × 4.6 mm i.d.) was used as a stationary phase. Separation was conducted by isocratic mode (mobile phase containing 70% [v/v] methanol and 30% [v/v] water). Sample introduction was performed through a 20 μL loop assembled to a Rheodyne 7725i six-port manual injector (Rohnert Park, CA, USA).
The concentration values obtained from these techniques were corrected according to the dilutions of the acceptor phase that took place after removing each sample. This correction was made using the following equation:
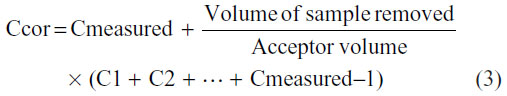
where Ccor corresponds to the corrected concentration; Cmeasured corresponds to the concentration measured in the acceptor at time t; C1, C2, and Cmeasured − 1 correspond to the measured concentrations at time 1, 2, and t − 1, respectively.
After this correction, the penetration percentage was calculated with the following equation:
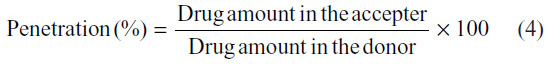
Statistical analysis
For the particle size, surface charge, and loading efficiency data, one-way analysis of variance (ANOVA) was performed to compare multiple groups. Differences between groups were compared with a post hoc test (Tukey’s honestly significant difference). Results are reported as mean ± standard deviation from a minimum of three independent experiments. Differences were considered significant at P < 0.05 or P < 0.001. All statistical analyses were performed with the software PASW Statistics 20 (IBM Corporation, Armonk, NY, USA).
Results and discussion
Particle size, surface charge, morphology, and lipid polymorphism
For the characterization of NLCs, particle size and surface charge (zeta potential) were analyzed and the results (Figure 1) show that nanoparticles loaded with both drugs (minoxidil and finasteride) present a mean size and PI of almost 200 nm and below 0.250, respectively, as desired to achieve delivery to the dermis and hair follicles. The polydispersity values obtained, which were small (<0.250), suggest a fairly narrow and monomodal particle size distribution. It is possible to observe that the NLCs with minoxidil (NLCs B and C) had a larger size than those without the drug (NLC A). Thus, minoxidil possibly has an influence on the size of the particles. However, within minoxidil-loaded nanoparticles, the size did not seem to change with different drug concentrations of the drug. Probably if the drug were distributed by adsorption on the surface of the nanoparticle, the effect on particle size would be more evident.19 Nevertheless, after loading finasteride (NLCs E and F), the mean particle size did not increase or decrease significantly. This indicates that the incorporation of this drug does not have a great influence on nanoparticle size. The slight differences observed in the nanoparticle sizes could also be due to the size variability of the formulation, due to the manual method of production.
The results obtained from zeta potential measurements (Figure 2) reveal that the NLCs were negatively charged. According to the literature, when the absolute zeta potential value is higher than 30 mV for colloidal formulation, the particles are likely to be electrochemically stable under the storage condition because the surface charge prevents aggregation of the particles.17,18 All anti-alopecia-drug-loaded NLCs presented charges around −30 mV, which indicates that the nanoformulations in this study had good physical stability. These values suffered variations with the presence of the anti-alopecia drugs (samples B, C, E, and F) compared with the free nanoparticles (samples A and D, respectively). However, the zeta potential was affected by the drug encapsulation in minoxidil-loaded NLCs, but not significantly affected in finasteride-loaded NLCs.
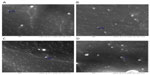
To obtain more information about the particle size and morphology, SEM analysis was also performed. All nanoparticles exhibited a spherical shape (Figure 3) and a smooth surface, regardless of their composition. The sizes of the nanoparticles observed by SEM were shown to be similar to those obtained by DLS; however, it has been reported that solvent removal may cause modification changes that can influence particle shape and size.20 Points that can be seen on the images obtained are probably due to the coating.
DSC relies on the fact that different lipid modifications possess different melting points, as have been performed to characterize the polymorphism and the degree of crystallinity of the LNs. Table 2 presents the results obtained for the DSC parameters of the nanoformulations and their bulk materials. Bulk mixture 1 has cetyl palmitate, oleic acid, and polysorbate 60; bulk mixture 2 has Precirol ATO 5, Miglyol 812, and polysorbate 60. Despite the enthalpy obtained for the bulk lipids being much higher than that of NLCs, meaning that there is a loss of crystallinity of the lipids after their incorporation into NLCs, the lipids in these formulations still possess some degree of crystallinity, as observed by the presence of β-form in the DSC thermograms (Figure S1). Indeed, the melting enthalpy in NLCs free of drug decreased from 150.43 J/g (bulk mixture 1) to 11.43 J/g for the first formulation and from 18.40 J/g (bulk mixture 2) to 0.85 J/g for the second formulation, indicating a slower recrystallization and a crystal order disturbance that might influence the loading capacity. Also, enthalpy of bulk mixtures decreased relative to the corresponding solid lipids. This was expected to happen, since oil incorporation lowers the crystallization and melting temperatures of the particle matrix and accelerates the transition of the solid lipid into the stable β-polymorph after crystallization.21 Additionally, surfactants distributed in the melted lipid phase can interfere with lipid crystallization, resulting in a lower melting enthalpy. Generally, the presence of guest molecules in the lipid matrix also influences its crystallization degree, decreasing the lipid layer organization.10 Thus, the enthalpy of NLCs also decreased with the presence of the drugs. Furthermore, the RI of NLCs was also lower in the presence of drug (samples NLC B and F). Solid state was confirmed for both body (37°C) and room (25°C) temperatures, since the onset temperature and the melting peak were well above these temperatures. The unstable lower melting modification (α-polymorphic form) observed in the melting curves of cetyl palmitate and Precirol ATO 5 was not observed in drug-loaded NLCs (Figure S1). This was caused by the fact that polymorphic transitions can occur more rapidly in NLCs than in bulk state.22 On the other hand, the DSC measurements were not done on the same day of NLC production, but some days after. During storage, the peak corresponding to the α-modification becomes smaller, which can also explain the fact that the lower melting modification was not observed. For NLC formulations of minoxidil, two maximum peaks occurred, revealing that the drug does not dissolve well in the lipid matrix.23
Evaluation of NLC stability
To assess the stability of the formulations developed, particle size, surface charge, and loading efficiency were measured over 28 days.
Particle size
Figure 4 shows the mean particle size of anti-alopecia-drug-loaded NLCs evaluated at different times over 28 days of investigation. The results show that, despite some variations, mean particle size and PI were kept constant over the observation period for both drugs tested, and all formulations (both loaded and unloaded) presented particles with sizes that were in the range of 131 to 192 nm for minoxidil, and 179 to 240 nm for finasteride. The nonsignificant size variations observed were due to the manual procedure of LN production that can introduce some errors: the movements of the arm and the positions of the tubes regarding the ULTRA-TURRAX and sonication tips were not exactly the same every time the formulations were produced, although an effort was made to eliminate these variations. The mean size and PI of all formulations were, over 28 days, almost 200 nm and below 0.250, respectively, as desired for dermal application. These findings suggest a good physical stability of both nanosystems loaded with anti-alopecia drugs, over 28 days of storage, which make them appropriate to use in alopecia treatment.
Zeta potential
Regarding the determination of the zeta potential, the results of which are presented in Figure 5, it is possible to observe that these values were always negative and around −30 mV. For minoxidil formulations, there seemed to be a slight tendency toward increased zeta potential absolute value when concentration of drug increased.
Over 28 days, there were no evident changes of the zeta potential values; indeed, all zeta potential values remained around 30 mV, in absolute value. These outcomes reveal that anti-alopecia-drug-loaded NLCs present good physical stability in storage over 28 days, and that particle aggregation and coalescence are unlikely to occur due to the high electrostatic repulsion between the LNs. Hence, these findings, as with those for size measurements, suggest good stability of nanocarrier systems.
Loading efficiency
Table 3 shows the results of the anti-alopecia drugs’ loading efficiency over 28 days of investigation. Percentages obtained for minoxidil NLCs on the day of production show that a small quantity of drug was incorporated. Minoxidil is a hydrophilic drug – a behavior that does not favor the interaction of this drug with lipids – which explains these results. Over 28 days, there was a slight (nonsignificant) decrease in these percentages, presumably, again, due to minoxidil’s hydrophilic character that explains the drug release into the aqueous media. As such, in spite of the low incorporated quantities, minoxidil loaded-NLCs were found to have good chemical stability. Since finasteride is hydrophobic, it explains that this drug has high loading on lipid nanoparticles, and also that over 28 days it does not escape from nanoparticles. Therefore, the high loading values remained constant over 28 days. As such, finasteride-loaded NLCs were found to have good chemical stability.
In vitro controlled release studies
To evaluate the mechanism of anti-alopecia drug release from NLCs, an in vitro release using a dialysis bag was performed. The results obtained for minoxidil, for the simulated physiological conditions of plasma (37°C and pH 7.4), are presented in Figure 6. From the results it is possible to observe a low and continuous release of minoxidil from the LNs, which is consistent with sustained drug-delivery systems.
  | Figure 6 Amount of minoxidil release (%) from the dialysis bag containing NLC B or NLC C, phosphate buffer at pH 7.4, at 37°C. |
However, for finasteride, under the experimental conditions adopted, the release profile was not found, since the drug was not released after 24 hours. That is, the drug was not released from the nanoparticles as it is very lipophilic. This is not a problem in NLC application on skin, because, after the nanoparticle penetration, the drug will eventually be released or the nanoparticle will be degraded (eg, by enzymes24) and, consequently, the drug will not be loaded in nanoparticles.
The tested controlled release in aqueous conditions, with physiological values of pH and temperature, proved that NLCs loaded with minoxidil yield a prolonged release of the drug from the solid lipid matrix of the nanoparticles. In conclusion, as release is reduced, both drugs remain in the nanoparticles and therefore will penetrate the skin encapsulated in NLCs, which is an advantage. A prolonged release is of interest for drugs encapsulated in cosmetics that will thus have a more sustained and prolonged effect.8
In vitro penetration studies
Anti-alopecia-drug-loaded NLCs were studied using Franz diffusion cells in order to assess the penetration of drugs through the skin. Results for minoxidil are presented in Figure 7. A second-order polynomial fit was used to draw the profile of penetration. After 24 hours, only a very small quantity of minoxidil crossed the skin. A previous study1 indicated the difficulty that exists in this drug penetrating through the skin. Therefore, the results obtained in this work were not different than what was expected, according to the literature. A possible way to promote minoxidil penetration is with the application of permeation enhancers, such as alcoholic solutions.
  | Figure 7 Penetration of minoxidil-loaded NLCs through the skin over 24 hours. |
Finasteride penetration was assessed using HPLC instead of spectrophotometer UV-VIS – thereby circumventing the problems related to the interference of the skin components released during the experiment. As only a small amount of finasteride crossed the skin (ie only a small amount was released from NLCs that crossed the skin), concentration values lower than the limit of quantification of HPLC technique were obtained for most of the samples. Still, for the last samples, the values obtained were quantifiable – after 24 hours, the mean penetration percentage for NLC E was 0.050%, and for NLC F was 0.004%. This difference between NLC E and NLC F could be due to the fact that the skin used for both experiments was from different ears and pigs, which introduces variance. These results show that only a very small quantity of finasteride crossed the skin, which is in accordance with a previous study.13 Therefore, reduced penetration was expected and so the results obtained in this work were low, but although only a small quantity of drug crossed the skin, other studies also obtained very small quantities, so this result is substantiated.
Conclusion
The success of dermatological products in alopecia therapy depends on the capability of the drug to overcome the stratum corneum and to penetrate through skin in sufficient amounts to achieve the desired therapeutic effect. In this work, NLCs were chosen as systems with the ability to incorporate and deliver anti-alopecia drugs into the dermis and hair follicles.
LNs with anti-alopecia drugs were produced successfully by ultrasonication method. It was possible to obtain NLCs with a size around 200 nm, as desired. Zeta potential values, in absolute value, were high enough to assure a good electrostatic stabilization for all NLCs tested. Over 28 days of investigation, a constant particle size could be observed and zeta potential values stayed practically unchanged – results that indicate good physical stability in storage. Good chemical stability of anti-alopecia-drug-loaded NLCs was also observed when stored for 28 days, in that loading efficiency remained constant. This is important to avoid losing drugs during production. Morphological investigations showed that all nanoparticles exhibited a spherical shape and a smooth surface independent of their composition. Furthermore, DSC investigations showed that all loaded NLCs were solid at both body (37°C) and room (25°C) temperatures, since the onset temperature and the melting peak were well above these temperatures, as desired for dermal application. Controlled release assays demonstrated that, with physiological values of pH and temperature, NLCs loaded with minoxidil yielded a prolonged release of the drug from the solid lipid matrix of the nanoparticles. This type of release is of interest for drugs encapsulated in cosmetics that will thus have a more sustained and prolonged effect. After 24 hours, finasteride was not released from nanoparticles, as it is very lipophilic. Penetration assays demonstrated that, when physiological values of pH and temperature were mimicked, NLCs loaded with minoxidil and finasteride had low levels of penetration through pig ear skin. Finasteride presented some problems in detection and measurement; such problems could be bypassed by improving quantification/detection limits of the method.
Before concluding about the real potentiality of these nanoformulations and the possibility of actually using these NLCs in anti-alopecia therapy, several experiments still need to be performed. DSC investigations should be done over time to evaluate the possible polymorphic modifications that can occur in storage. Furthermore, despite this work involving various attempts to achieve minoxidil loaded NLCs with good loading efficiency, future work could be focused on the best way to incorporate minoxidil in LNs, ensuring higher loading efficiency. Further, the addition of another drug to load should be considered, in order to create more effective NLCs, and therefore more ways of action. Nevertheless, the combination of these two anti-alopecia drugs, minoxidil and finasteride, incorporated in NLCs, has proved to have several good characteristics that indicate that this proposed novel formulation can be an excellent therapy for alopecia.
Acknowledgments
The authors are thankful to Professor Dr Amália Jurado and Dr Catarina Morais (CNC, UC) for help with DSC experiments. We acknowledge the Fundação para a Ciência e a Tecnologia for the financial support through Strategic Project PEst-C/EQB/LA0006/2011. We are also thankful to Dr Daniela Silva (CEMUP, UP) for expert help with SEM.
Disclosure
The authors report no conflicts of interest in this work.
References
Padois K, Cantiéni C, Bertholle V, Bardel C, Pirot F, Falson F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int J Pharm. 2011;416:300–304. | |
Ellis JA, Sinclair RD. Male pattern baldness: current treatments, future prospects. Drug Discov Today. 2008;13:791–797. | |
Wosicka H, Cal K. Targeting to the hair follicles: current status and potential. J Dermatol Sci. 2010;57:83–89. | |
Alzolibani AA. Epidemiologic and genetic characteristics of alopecia areata (part 1). Acta Dermatovenerol Alp Panonica Adriat. 2011;20(4):191–198. | |
Coda AB, Sinha AA. Integration of genome-wide transcriptional and genetic profiles provides insights into disease development and clinical heterogeneity in alopecia areata. Genomics. 2011;98(6):431–439. | |
Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 2010;27(7):247–259. | |
Chourasia R, Jain SK. Drug targeting through pilosebaceous route. Curr Drug Targets. 2009;10(10):950–967. | |
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–184. | |
Torchilin VP, editor. Nanoparticulates as Drug Carriers. London: Imperial College Press; 2006; Chapter 9. | |
Schäfer-Korting M, editor. Drug Delivery. Berlin: Springer-Verlag; 2010;115–143. | |
Kwon TK, Kim JC. In vitro skin permeation of monoolein nanoparticles containing hydroxypropyl beta-cyclodextrin/minoxidil complex. Int J Pharm. 2010;392(1–2):268–273. | |
Souto EB, Müller RH. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int J Cosmet Sci. 2008;30(3):157–165. | |
Tabbakhian M, Tavakoli N, Jaafari MR, Daneshamouz S. Enhancement of follicular delivery of finasteride by liposomes and niosomes 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. Int J Pharm. 2006;323(1–2):1–10. | |
Ricci M, Puglia C, Bonina F, Giovanni CD, Giovagnoli S, Rossi C. Evaluation of indomethacin percutaneous absorption from nanostructured lipid carriers (NLC): in vitro and in vivo studies. J Pharm Sci. 2005;94(5):1149–1159. | |
Goldman MP, Bacci PA, Leibaschoff G, Hexsel D, Angelini F. Cellulite: Pathophysiology and Treatment. New York: Taylor and Francis Group; 2006. | |
Al-Bader T, Davis M, Laloeuf A, Rawlings AV, inventors; Oriflame Cosmetics SA, assignee. Topical cosmetic compositions for treating or preventing cellulite. Patent EP2009/054043. April 3, 2009. | |
Mohanraj V, Chen Y. Nanoparticles – a review. Tropical Journal of Pharmaceutical Research. 2006;5(1):561–573. | |
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. | |
Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59:427–443. | |
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. | |
Saupe A, Wissing SA, Lenk A, Schmidt C Müller RH. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) – structural investigations on two different carrier systems. Biomed Mater Eng. 2005;15(5):393–402. | |
Bunjes H, Koch MH, Westesen K. Influence of emulsifiers on the crystallization of solid lipid nanoparticles. J Pharm Sci. 2003;92(7):1509–1520. | |
Zimmermann E, Souto EB, Müller RH. Physicochemical investigations on the structure of drug-free and drug-loaded solid lipid nanoparticles (SLN) by means of DSC and 1HNMR. Pharmazie. 2005;60(7):508–513. | |
Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349–358. |
Supplementary figure
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.