Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Is hypertriglyceridemia a prognostic factor in sepsis?
Authors Cetinkaya A , Erden A, Avci D , Karagoz H, Karahan S, Basak M, Bulut K, Gencer V, Mutlu H
Received 18 November 2013
Accepted for publication 6 January 2014
Published 27 February 2014 Volume 2014:10 Pages 147—150
DOI https://doi.org/10.2147/TCRM.S57791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ali Cetinkaya,1 Abdulsamet Erden,1 Deniz Avci,1 Hatice Karagoz,1 Samet Karahan,1 Mustafa Basak,1 Kadir Bulut,1 Vedat Gencer,1 Hasan Mutlu2
1Internal Medicine Department, Kayseri Training and Research Hospital, Kayseri, Turkey; 2Medical Oncology Department, Acibadem Kayseri Hospital, Kayseri, Turkey
Introduction: Sepsis and septic shock are important causes of mortality in intensive care unit patients, hence early diagnosis and therapy are important in management of their treatment. The available information on sepsis patients is not enough to recommend or to discard the routine evaluation of triglyceride (TG) levels at the onset of sepsis. The aim of this study was to investigate the association of hypertriglyceridemia and clinical outcome (or mortality) in patients with severe sepsis.
Materials and methods: Between January 1 and December 31, 2011, a total of 84 patients with sepsis from the intensive internal care unit at the Kayseri Training and Research Hospital, Kayseri, Turkey, were investigated retrospectively. Sepsis was defined according to the American College of Chest Physicians/Society of Critical Care Medicine/European Society of Intensive Care Medicine consensus conference definitions. For each patient, survival was recorded at the end of the last day of hospitalization as dead or alive. The TG values were taken retrospectively from the records, which were performed routinely for each patient with sepsis at the time of diagnosis. TG >150 mg/dL was considered as hypertriglyceridemia.
Results: The percentages of male and female patients were 44% and 56%, respectively. The mean age of patients was 71.49±11.071 years. The percentage of patients with TG values more than 150 mg/dL was 81% (25/31) in the non-survivor group and 19% (6/31) in the survivor group. There was a significant difference regarding TG values between groups (P=0.039).
Discussion: It was observed in this study that patients in the intensive care unit with sepsis had high TG levels. We also observed that the TG level >150 mg/dL at 0 hour (onset of sepsis) was a significant predictive marker of sepsis mortality rate. The contribution of hypertriglyceridemia to mortality might be modest compared to increase in severity of illness, but, nevertheless, these simple measurements represent a potential therapeutic target in sepsis.
Keywords: triglyceride, prognosis, mortality, sepsis
Introduction
Sepsis and septic shock are important causes of mortality1 in intensive care unit patients, hence early diagnosis and therapy are important in management of their treatment. Sepsis is defined as the association of a number of nonspecific inflammatory responses with evidence, or suspicion, of a microbial origin.2,3 The signs and symptoms of sepsis are highly variable. Symptoms are nonspecific but present a picture of systemic illness. Lipopolysaccharide, which is released from the outer membrane of Gram-negative bacteria, plays a key role in initiating the inflammatory response in sepsis. Hypertriglyceridemia is commonly observed in patients with Gram-negative infections and animals administered live Gram-negative bacteria or endotoxin.4 In one study, the elevation in plasma triglyceride (TG) level observed 24 hours after Escherichia coli infusion was attributed to a reduction in TG clearance rates rather than an increase in liver secretion.5 Increased plasma TG concentrations can result from an increased rate of very-low-density lipoprotein (VLDL) secretion from the liver, from a decreased rate of removal of VLDL and TG, or from a combination of changes in both processes. Many of the sepsis-induced disturbances in lipid metabolism are thought to be cytokine related. Tumor necrosis factor (TNF)-α has been shown to elevate serum TG level by increasing the hepatic production6,7 and also decreasing lipoprotein lipase (LPL) activity in adipose tissue.8,9 The available information on sepsis patients is not enough to recommend or to discard the routine evaluation of TG levels at the onset of sepsis. The aim of this study was to investigate the association of hypertriglyceridemia and clinical outcome (or mortality) in patients with severe sepsis.
Materials and methods
Between January 1 and December 31, 2011, a total of 84 patients with sepsis from the intensive internal care unit at the Kayseri Training and Research Hospital, Kayseri, Turkey, were investigated retrospectively, using hospital records. Medical history was recorded from each patient’s chart, including age, sex, and chronic background illnesses (ischemic heart disease, diabetes mellitus, hypertension, malignancy, renal failure). Blood pressure, heart rate, and body temperature were recorded for each patient. Laboratory data included: complete blood count; urea; creatinine; glucose; calcium; sodium; potassium; phosphate; uric acid; liver function tests; albumin; and a lipid profile (total cholesterol, calculated serum high-density lipoprotein cholesterol, and TG). Sepsis was defined according to the American College of Chest Physicians/Society of Critical Care Medicine/European Society of Intensive Care Medicine consensus conference definitions.The patients included in this study were on broad-spectrum antibiotics and were not taking lipid-lowering drugs. Patients who received enteral or parenteral nutrition within 8 hours before the diagnosis of sepsis were excluded.Those with a history of chronic renal failure, diabetes mellitus, ischemic heart disease, hypertension, or hyperlipidemia were excluded. For each patient, survival was recorded at the end of the last day of hospitalization as dead or alive. The TG values were taken retrospectively from the records, which were performed routinely for each patient with sepsis at the time of diagnosis. At our institution’s laboratory, the upper limit for serum TG value is 149 mg/dL. In general, serum TG concentration levels less than 150 mg/dL (less than 1.7 mmol/L) are accepted as normal; 150 to 199 mg/dL (1.7 to 2.2 mmol/L) as close to high; 200 to 499 mg/dL (2.3 to 5.6 mmol/L) as high; and greater than 500 mg/dL (5.7 mmol/L) as very high.10 Thus, before data analysis, hypertriglyceridemia was defined as any TG level ≥150 mg/dL.
Statistical analysis
For statistical analysis, the Statistical Package for the Social Sciences ([SPSS] v 16; IBM Corporation, Armonk, NY, USA) was used. Continuous variables were tested for normal distribution by the Kolmogorov–Smirnov test. We reported continuous data as mean and standard deviation. Categorical variables were summarized as percentages and compared with the chi-square test. The chi-square test was used to evaluate the relationship between TG levels and mortality. P-values<0.05 were considered significant. To evaluate the relationship between biochemical parameters and mortality, independent samples t-test was used. In addition, mean and frequency analysis were used.
Results
The percentages of male and female patients were 44% and 56%, respectively. The mean age of patients was 71.49±11.071 years. Considering the patients as survivors or non-survivors, the mean age of patients was 68.19±13.88 years in the survivor group and 72.97±9.31 years in the non-survivor group (P=0.019). When biochemical parameters were evaluated, no significant difference was found between survivor and non-survivor groups. The mean TG level of patients was 122.96±65.01 mg/dL in the survivor group and 167.42±109.58 mg/dL in the non-survivor group (P=0.083). The relationship of biochemical parameters and mortality in patients with sepsis is given in Table 1. TG >150 mg/dL was considered as hypertriglyceridemia. The percentage of patients with TG values more than 150 mg/dL was 81% (25/31) in the non-survivor group and 19% (6/31) in the survivor group. For the TG values, there was a significant difference between the two groups (P=0.039). Values for each group are given in Table 2. The risk of mortality in the group of patients with high TG levels was 81%, compared to 58% in the group with normal TG levels. The odds for mortality were 4.1 (25/6) and 1.4 (28/20), respectively. We calculated and added the risk ratio (0.806/0.583=1.382) and the odds ratio (4.1/1.4=2.928).
  | Table 1 The relationships between biochemical parameters and mortality in patients with sepsis |
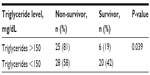  | Table 2 The relationship between triglyceride levels and mortality in patients with sepsis |
Discussion
In this study, it was observed that patients in the intensive care unit with sepsis had high TG levels. Lipoproteins have been known to play a role in innate immunity and variations in their levels have been observed in a variety of inflammatory disorders, but not much is known about lipoprotein metabolism in patients with sepsis. Elevated plasma TG level may result either from a decreased rate of peripheral removal or an increased rate of production and secretion of liver. Previous data, showing a reduction in LPL activity in peripheral tissues of Gram-negative septic rats11–13 and septic patients,14 suggest a defect in the clearance of TG as one of the factors involved in the cause of hypertriglyceridemia. The observed decrease in tissue LPL activity in rats administered live E. coli was also expected, since postheparin plasma lipase activity and adipose tissue LPL activity have been reported to be decreased in bacteremic monkeys and rats, respectively.15,16 Concomitant with the reduced enzyme activities, circulating TG concentrations were increased. The initial rise in serum TG level in the E. coli-treated rats may have been mediated by the increased concentrations of plasma catecholamines and glucocorticoids, which are also elevated in rat models of endotoxemia,17,18 polymicrobial sepsis,19 and E. coli bacteremia.20 Recently, catecholamines have been shown to mediate endotoxin-induced hypertriglyceridemia, may contribute to the downregulation of adipose LPL.21 Epinephrine has also been shown to lower LPL activity in adipocytes by way of decreasing LPL synthesis.22 Cytokines such as TNF-α and the interleukins may mediate the hypertriglyceridemia associated with sepsis and endotoxemia.23 A study on endotoxin-injected rats with suppressed LPL tissue activities showed that the hypertriglyceridemia was the result of a reduction in the clearance rate of TG from the plasma.24 Endotoxemia25 and polymicrobial sepsis26 have also been shown to have produced an elevation in serum TNF-α at the 60th minute. TNF administration was shown to produce a rapid rise in serum TG level between the 45th and 90th minutes, which was due to an increase in hepatic TG synthesis and VLDL secretion.27,28 Other cytokines, such as interferons, the interleukins, and platelet-activating factor, may also have a role in altering lipid metabolism after E. coli sepsis.29,30 One study showed that the hypertriglyceridemia of sepsis may have a protective function.31 Lipoproteins bind endotoxin, thus preventing their interaction with lipopolysaccharide-binding protein and, subsequently, their uptake by macrophages that triggers the release of the inflammatory mediators.31 We have also observed that the TG level >150 mg/dL at 0 hour (onset of sepsis) was a significant predictive marker of sepsis mortality rate, and hypertriglyceridemia can be defined as a risk factor for mortality in patients with sepsis.
The contribution of hypertriglyceridemia to mortality might be modest compared to increase in severity of illness, but, nevertheless, these simple measurements represent a potential therapeutic target in sepsis. The mechanism that modifies TG level in sepsis is not yet well understood, and further studies in larger populations with sequential cholesterol monitoring for a longer period will lead to more timely interventions and enhance patient outcomes.
Conclusion
According to our results, the routine measurement of serum TGs at the onset of sepsis and sequential monitoring are recommended. The measurement of TG can be a good predictor of hospital death in sepsis ; however, from the observations of the present study, we suggest that new therapies directed at decreasing serum TG levels may offer important alternative options for better management of sepsis.
Disclosure
The authors have no conflicts of interest to report in this work.
References
Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–123. | |
[No authors listed]. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. | |
Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. | |
Scholl RA, Lang CH, Bagby GJ. Hypertriglyceridemia and its relation to tissue lipoprotein lipase activity in endotoxemic, Escherichia coli bacteremic, and polymicrobial septic rats. J Surg Res. 1984;37(5):394–401. | |
Lanza-Jacoby S, Tabares A. Triglyceride kinetics, tissue lipoprotein lipase, and liver lipogenesis in septic rats. Am J Physiol. 1990; 258(4 Pt 1):E678–E685. | |
Feingold KR, Staprans I, Memon RA, et al. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res. 1992;33(12):1765–1776. | |
Feingold KR, Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987;80(1):184–190. | |
Grunfeld C, Gulli R, Moser AH, Gavin LA, Feingold KR. Effect of tumor necrosis factor administration in vivo on lipoprotein lipase activity in various tissues of the rat. J Lipid Res. 1989;30(4):579–585. | |
Semb H, Peterson J, Tavernier J, Olivecrona T. Multiple effects of tumor necrosis factor on lipoprotein lipase in vivo. J Biol Chem. 1987;262(17):8390–8394. | |
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. | |
Bagby GJ, Spitzer JA. Lipoprotein lipase activity in rat heart and adipose tissue during endotoxic shock. Am J Physiol. 1980;238(3):H325–H330. | |
Bagby GJ, Spitzer JA. Decreased myocardial extracellular and muscle lipoprotein lipase activities in endotoxin-treated rats. Proc Soc Exp Biol Med. 1981;168(3):395–398. | |
Lanza-Jacoby S, Tabares A, Sitren HS, Kosar E. Comparison of glucose and glucose plus lipid as caloric sources in parenterally fed rats. Am J Physiol. 1987;253(2 Pt 1):E158–E164. | |
Robin AP, Greenwood MR, Askanazi J, Elwyn DH, Kinney JM. Influence of total parenteral nutrition on tissue lipoprotein lipase activity during chronic and acute illness. Ann Surg. 1981;194(6):681–686. | |
Kaufmann RL, Matson CF, Rowberg AH, Beisel WR. Defective lipid disposal mechanisms during bacterial infection in rhesus monkeys. Metabolism. 1976;25(6):615–624. | |
Lanza-Jacoby S, Lansey SC, Cleary MP, Rosato FE. Alterations in lipogenic enzymes and lipoprotein lipase activity during gram-negative sepsis in the rat. Arch Surg. 1982;117(2):144–147. | |
McKechnie K, Dean HG, Furman BL, Parratt JR. Plasma catecholamines during endotoxin infusion in conscious unrestrained rats: effects of adrenal demedullation and/or guanethidine treatment. Circ Shock. 1985;17(1):85–94. | |
Givalois L, Dornand J, Mekaouche M, et al. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–R170. | |
Hahn PY, Wang P, Tait SM, Ba ZF, Reich SS, Chaudry IH. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock. 1995;4(4):269–273. | |
Jones SB, Westfall MV, Sayeed MM. Plasma catecholamines during E. coli bacteremia in conscious rats. Am J Physiol. 1988;254(3 Pt 2):R470–R477. | |
Nonogaki K, Moser AH, Feingold KR, Grunfeld C. Alpha-adrenergic receptors mediate the hypertriglyceridemia induced by endotoxin, but not tumor necrosis factor, in rats. Endocrinology. 1994;135(6):2644–2650. | |
Ong JM, Saffari B, Simsolo RB, Kern PA. Epinephrine inhibits lipoprotein lipase gene expression in rat adipocytes through multiple steps in posttranscriptional processing. Mol Endocrinol. 1992;6(1):61–69. | |
Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986;320(6063):584–588. | |
Bagby GJ, Corll CB, Martinez RR. Triacylglycerol kinetics in endotoxic rats with suppressed lipoprotein lipase activity. Am J Physiol. 1987;253(1 Pt 1):E59–E64. | |
Cannon JG, Tompkins RG, Gelfand JA, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161(1):79–84. | |
Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor). Circ Shock. 1992;36(3):191–199. | |
Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–664. | |
Chajek-Shaul T, Friedman G, Stein O, Shiloni E, Etienne J, Stein Y. Mechanism of the hypertriglyceridemia induced by tumor necrosis factor administration to rats. Biochim Biophys Acta. 1989;1001(3):316–324. | |
Martich GD, Danner RL, Ceska M, Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173(4):1021–1024. | |
Evans RD, Ilic V, Williamson DH. Metabolic effects of platelet-activating factor in rats in vivo. Stimulation of hepatic glycogenolysis and lipogenesis. Biochem J. 1990;269(1):269–272. | |
Harris HW, Grunfeld C, Feingold KR, Rapp JH. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990;86(3):696–702. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
