Back to Journals » Clinical Ophthalmology » Volume 9
Indications and postoperative treatment for Ex-PRESS® insertion in Japanese patients with glaucoma: comparison with standard trabeculectomy
Authors Kato N, Takahashi G, Kumegawa K, Kabata Y , Tsuneoka H
Received 13 April 2015
Accepted for publication 11 June 2015
Published 18 August 2015 Volume 2015:9 Pages 1491—1498
DOI https://doi.org/10.2147/OPTH.S86504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Noriko Kato,1,2 Genichiro Takahashi,1,2 Koichi Kumegawa,1,2 Yoshiaki Kabata,1,2 Hiroshi Tsuneoka1
1Department of Ophthalmology, Jikei University, School of Medicine, 2Katsushika Medical Center, Tokyo, Japan
Background: We investigated indications and early postoperative treatment for Ex-PRESS® insertion for glaucoma by comparing postoperative outcomes with those for standard trabeculectomy.
Methods: Ex-PRESS insertion was performed in 21 eyes and standard trabeculectomy (TLE) in 22 eyes. Mean intraocular pressure (IOP) in the 6 months after surgery, success rate for postoperative IOP decline, postoperative complications, postoperative treatment, filtering blebs, and indications were then retrospectively investigated.
Results: Mean postoperative IOP did not differ significantly between the groups at any observation time for 6 months after surgery. Further, it did not differ between either the groups of patients with primary open-angle glaucoma (POAG) and neovascular glaucoma (NTG), or the patients with primary open-angle glaucoma and NTG in the Ex-PRESS group. Comparison of success rates in reduction of postoperative IOP between the groups under the following four survival conditions showed no significant differences: postoperative IOP <30% of the preoperative IOP, complete success (no additional ophthalmic solution), and qualified success (ophthalmic solution required); 5 mmHg ≤ postoperative IOP ≤21 mmHg, complete success (no additional ophthalmic solution), and qualified success (ophthalmic solution required). With regard to postoperative complications and postoperative treatment, the incidence of hyphema was significantly lower in the Ex-PRESS group, but no other significant intergroup differences were seen. The height of the filtering bleb was lower in the Ex-PRESS group.
Conclusion: Postoperative outcomes in the Ex-PRESS and TLE groups were comparable. The incidence of hyphema was significantly lower in the Ex-PRESS group. Ex-PRESS insertion appears to be useful in patients with NTG and in those prone to postoperative bleeding. There were no significant intergroup differences in postoperative treatment. Assessment of outcome after Ex-PRESS insertion was difficult in some patients. Postoperative treatment should be developed to suit the specific requirements of Ex-PRESS insertion.
Keywords: Ex-PRESS, trabeculectomy, indications, neovascular glaucoma, postoperative treatment
Introduction
Trabeculectomy (TLE) is a surgical filtering method that is widely used in the treatment of glaucoma. Although outcomes after TLE can be improved by combination with mitomycin C (MMC) or other anticancer drugs, TLE may nevertheless result in a shallow anterior chamber, hyphema, hypotony maculopathy, postoperative endophthalmitis, and other complications,1 and may result in impaired visual function. Various measures to prevent or minimize these risks have been introduced, but results to date have been insufficient.
The Ex-PRESS® glaucoma filtration device (hereinafter “Ex-PRESS”; Alcon Laboratories, Fort Worth, TX, USA) is a stainless steel filtration device. This device creates a scleral flap in the same way as standard TLE and makes a puncture from the corneal limbus to the anterior chamber.2 The aqueous humor is drained through the device to the subconjunctival space, which reduces the intraocular pressure (IOP). Outcomes after Ex-PRESS insertion are reported to be equivalent to those after standard TLE.3–8 Ex-PRESS insertion seldom causes a shallow anterior chamber intraoperatively and does not require sclerotomy or peripheral iridectomy, and is therefore considered a useful filtering surgical method with fewer complications. However, it is unclear whether Ex-PRESS insertion is equivalent to standard TLE with regard to indications and filtering bleb assessment.
Reported risk factors for the failure of Ex-PRESS insertion include diabetes, non-Caucasian race, and previous glaucoma surgery.9 However, candidates suitable for Ex-PRESS insertion and postoperative treatment requirements in Japanese patients with glaucoma have not been characterized.
Here, we retrospectively compared postoperative IOP, success rate for postoperative IOP decline, postoperative complications, and the height of the filtering bleb between Japanese glaucoma patients undergoing Ex-PRESS insertion and those undergoing TLE for 6 months after surgery. We then determined the indications and postoperative procedures for Ex-PRESS insertion and TLE.
Subjects and methods
Glaucoma surgery was performed on 43 eyes in 41 consecutive patients between January 2012 and December 2013 at the Department of Ophthalmology, Jikei University School of Medicine, Katsushika Medical Center. Ex-PRESS insertion was done in 21 eyes and standard TLE in 22 eyes.
Glaucoma was diagnosed based on the following criteria at screening: IOP ≥21 mmHg; glaucomatous change in the fundus, regardless of type or severity; and an abnormal visual field that corresponded to fundus findings. For the present study, we specifically selected subjects with a poor decrease in IOP after medication. The inclusion criterion was a persistent visual field defect despite medical treatment using a maximum tolerated dose. Patients with glaucoma who had undergone cataract or vitreous surgery were also included. Exclusion criteria were intracranial and general disorders considered to have affected the visual field; glaucoma surgery within 6 months prior to study entry; inability or unwillingness to provide informed consent to treatment; no diagnosis of glaucoma; and lack of perimetry examination during screening.
Three surgeons (GT, KK, YK) explained the surgical method to the patients and obtained their informed consent. Randomization was achieved by assigning the eyes to the Ex-PRESS or TLE groups in an alternating fashion, including the two patients requiring surgery for both eyes, who were treated accordingly by both methods, one in each eye. The study was approved by the ethics committee of Jikei University School of Medicine and was conducted in accordance with the Declaration of Helsinki. The surgeries were performed as follows. For both groups, after topical anesthesia and sub-tenon local anesthesia with Xylocaine®, a fornix-based conjunctival flap was created, followed by a 50% depth 3×3 mm scleral flap dissected up to the clear cornea. A side port was created after application of 0.04% MMC for 3 minutes and washing out with an intraocular irrigation solution. For eyes randomized to Ex-PRESS, a pre-incision was made into the anterior chamber using a 25 G needle parallel to the iris, followed by implantation of the Ex-PRESS device. For eyes randomized to TLE, a sclerotomy was performed, followed by a peripheral iridectomy. The scleral flap was then sutured using 10-0 nylon for both groups, with the number of nylon sutures required depending on the degree of filtration. The conjunctiva was tightly sutured using 10-0 nylon. Postoperative treatment was provided in consideration of postoperative IOP, depth of the anterior chamber, and height of the filtering bleb.
IOP was measured using Goldmann applanation tonometry. IOP was measured by the attending physician at 8 am for hospitalized patients and midmorning for outpatients.
We then retrospectively compared the Ex-PRESS and TLE groups for postoperative IOP, postoperative complications, height of the filtering bleb, and postoperative treatment for 6 months after surgery.
Success rate for IOP decline was defined as either: the percentage of patients with a postoperative IOP that was <30% of the preoperative IOP; or the percentage of patients with a postoperative IOP between 5 mmHg and 21 mmHg. These two rates were compared between the groups with additional classification by whether postoperative IOP were achieved without the additional ophthalmic solution, or regardless of the additional ophthalmic solution. Thus, the rate of successful reduction in postoperative IOP was compared between the groups as follows: IOP <30%, complete success (no additional ophthalmic solution), qualified success (ophthalmic solution required); and 5 mmHg ≤ IOP ≤21 mmHg, complete success (no additional ophthalmic solution), and qualified success (ophthalmic solution required). Patients with IOP levels that did not achieve successful reduction on two consecutive visits were defined as having failed treatment. Regarding filtering bleb formation, the height of the filtering bleb was visually measured on the day after surgery and classified as flat, low, or high.
Statistical analyses
Statistical analyses were performed using R software 3224 (version 1.8.1, the R Foundation, http://www.r-project.org). Intergroup comparisons were made using the Mann–Whitney U-test and Fisher’s Exact test. The success rate was compared using the Kaplan–Meier method, and the survival curve using the log rank test. Differences were considered to be statistically significant when P-values were less than 5%.
Results
The patient characteristics are shown in Table 1. No intergroup difference was seen for any patient characteristics. Patient numbers by type of glaucoma are shown in Table 2. The majority of patients in both groups had primary open-angle glaucoma or neovascular glaucoma. After excluding patients with plateau iris, steroid-induced glaucoma, pseudoexfoliation glaucoma, or glaucoma after vitrectomy, we found no significant intergroup difference in the number of patients. Changes in mean (± standard deviation) postoperative IOP in the two groups are shown in Figure 1. IOP before and at 1 day, 1 week, 1 month, 2 months, 3 months, and 6 months after surgery was 29.9±7.8, 6.9±4.2. 8.7±4.7, 12.8±3.5, 14.7±5.2, 15.7±6.0 and 16.7±5.8 mmHg, respectively, in the Ex-PRESS group, and 31.9±10.3, 9.5±9.6, 10.6±9.0, 14.0±6.7, 12.9±5.1, 14.3±5.3, and 14.9±4.5 mmHg in the TLE group. No significant intergroup difference was seen at any observation time. Further, no significant intergroup difference in postoperative IOP was seen at any observation time when analysis was restricted to patients with primary open-angle glaucoma or neovascular glaucoma (Figures 2 and 3).
  | Table 1 Patient characteristics and study follow-up |
  | Table 2 Type of glaucoma |
In the Ex-PRESS group, there was no significant difference in postoperative IOP between patients with primary open-angle glaucoma or neovascular glaucoma (Figure 4).
When the success rate was defined as the percentage of patients with a postoperative IOP less than 30% of the preoperative IOP, there was no significant intergroup difference at any observation point. At 6 months after surgery, the complete success rate was 48.5% in the Ex-PRESS group and 53.0% in the TLE group (Figure 5) and the qualified success rate was 70.6% in the Ex-PRESS group and 71.40% in the TLE group (Figure 6). Moreover, when the success rate was defined as the percentage of patients with a postoperative IOP between 5 and 21 mmHg, there was no significant intergroup difference at any observation point. At 6 months after surgery, the complete success rate was 37.1% in the Ex-PRESS group and 45.5% in the TLE group (Figure 7) and the qualified success rate was 46.8% in the Ex-PRESS group and 71.40% in the TLE group (Figure 8).
Postoperative complications occurring in the 6 months after surgery are shown in Table 3. Hyphema was seen in two eyes in the Ex-PRESS group and in eleven eyes in the TLE group, with the difference being statistically significant (P=0.006). Hyphema in the two eyes in the Ex-PRESS group was caused by contact between the device and the iris associated with a shallow anterior chamber. The eleven eyes in the TLE group consisted of four eyes with neovascular glaucoma, two with primary open-angle glaucoma, two with uveitis glaucoma, and three with other types of glaucoma. The incidence of hyphema was higher in patients with neovascular glaucoma. Postoperative IOP was higher in four of the eleven eyes, and was treated with anterior chamber washout and IOP-lowering ophthalmic solutions. No significant intergroup difference was seen for other complications. In the Ex-PRESS group, contact between the device and the iris was seen in eight eyes (38.1%).
  | Table 3 Postoperative complications |
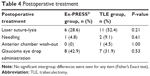
The postoperative treatment details are shown in Table 4. In the Ex-PRESS group, laser suture-lysis (LSL) was performed in four eyes within 1 week after surgery and in two eyes between 1 week and 1 month after surgery. In the TLE group, LSL was performed in nine eyes within 1 week after surgery and in two eyes between 1 week and 1 month after surgery. LSL was performed in more eyes in the TLE group, but the difference was not significant. Mean IOP when LSL was performed was 23.7 mmHg in the Ex-PRESS group and 22.0 mmHg in the TLE group, but again the difference was not significant.
  | Table 4 Postoperative treatment |
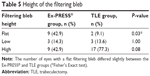
Regarding the height of the filtering bleb, the number of eyes with a flat filtering bleb differed significantly between the Ex-PRESS (nine eyes) and TLE groups (two eyes). In contrast, the number of eyes with a low or high filtering bleb did not significantly differ between the groups (Table 5).
Regarding the resumption of treatment for glaucoma with ophthalmic solutions (glaucoma eye drop administration), IOP at the start of treatment was 23.5 mmHg in the Ex-PRESS group and 29.9 mmHg in the TLE group, and the mean time interval between surgery and resumption of treatment was 61.6 days and 26.5 days, respectively. This difference in IOP at the resumption of treatment was not significant, whereas the difference in the number of days between surgery and resumption of treatment was significant.
In the Ex-PRESS group, postoperative complications and postoperative treatment did not differ significantly between patients with primary open-angle glaucoma or neovascular glaucoma (Table 6).
Discussion
In this study, we found no significant difference in the level of IOP decline, success rate, or postoperative treatment between patients undergoing Ex-PRESS insertion and TLE. Regarding postoperative complications, the incidence of hyphema was significantly lower in the Ex-PRESS group, as was the height of the filtering bleb on the day after surgery. These findings suggest that Ex-PRESS insertion is useful in patients with neovascular glaucoma and in patients who are prone to postoperative bleeding.
Previous studies reported that outcomes after Ex-PRESS insertion were comparable with those after TLE with MMC, which is the standard filtering surgical procedure, and that Ex-PRESS insertion was associated with fewer postoperative complications.3–8 De Jong et al reported that the success rate for Ex-PRESS insertion was significantly lower that for TLE during the first 3 years after surgery, but that rates were almost the same at 5 years.5 Sugiyama et al reported no significant intergroup difference in success rate at 24 months after surgery (approximately 80% for both groups),6 whereas Dahan et al reported a significantly higher success rate in the Ex-PRESS group (80%) than in the TLE group (40%).7 In a meta-analysis of four randomized clinical studies, the level of IOP decline was comparable between the two groups, whereas the success rate at 1 year after surgery was higher in the Ex-PRESS insertion group.8 Even though the success rate differed according to definition and length of observation, it was comparable between the two groups.
We consider that a consensus exists that IOP success when evaluating the effect of surgery for glaucoma should be reported with an upper and a lower limit. Given that normal IOP range is 10–21 mmHg and hypotony is likely when IOP is <5 mmHg, we therefore defined one survival condition as 5 mmHg ≤ IOP ≤21 mmHg. Although we did not precisely evaluate the severity of visual field damage and did not include normal-tension glaucoma, we considered the glaucoma damage in the enrolled subjects was moderate or greater, and accordingly defined an additional survival condition of IOP <30%.10–13
The success rate of reduction in postoperative IOP was compared between the Ex-PRESS and TLE groups under the following four survival conditions, regardless of postoperative treatment: at IOP <30%, complete success occurred when no additional ophthalmic solution was required, and qualified success occurred when ophthalmic solution was required; and at 5 mmHg ≤ IOP ≤21 mmHg, complete success occurred when no additional ophthalmic solution was required, and qualified success occurred when ophthalmic solution was required.
We considered that LSL and needling were suitable postoperative treatments for the re-establishment of bleb function and promotion of ongoing success. Further, a consensus on whether these treatments should be categorized as failures has not been obtained, and we did not record this as evidence of failure.
In the present study, we found no significant intergroup differences in the frequency of postoperative treatment. Further, we found no clear tendencies of the frequency of postoperative treatment, as in other reports.3–8 The method and timing of postoperative treatment are usually decided with consideration of IOP, anterior chamber depth, and filtering bleb height. We undertook postoperative treatment in the Ex-PRESS group based on that usually performed after TLE, but determining an appropriate method and timing was sometimes difficult because the height of the filtering bleb was lower in the Ex-PRESS group than in the TLE group during the early stage after surgery, as Good and Kahook have also reported.14 Height was low in nine of 21 patients in the Ex-PRESS group, and although IOP decreased without postoperative treatment in four of these nine patients, it was difficult to judge whether the filtering bleb could be maintained because its height was low. Reactions after LSL or ocular massage differed between the groups, and the height of the filtering bleb was lower in the Ex-PRESS group. In the TLE group, LSL was performed mainly when the IOP did not decrease sufficiently after ocular massage. The IOP after LSL was well controlled, but filtering blebs did not form sufficiently. When LSL was additionally performed, two patients developed excessive filtration and a shallow anterior chamber or hypotony. One advantage of Ex-PRESS is that its filtration rate can be kept constant. However, because the inner cavity of the device is narrow (50 μm), any increase in filtration rate after ocular massage will likely be smaller than in patients undergoing TLE. Further, because the filtration rate after Ex-PRESS insertion is constant, excessive filtration will likely be more difficult to control than in TLE. Thus, the timing of LSL and the point at which sutures should be cut require careful consideration. Regarding the resumption of treatment with ophthalmic solutions, we saw no significant intergroup difference in IOP on resumption of treatment, but the timing of resumption differed greatly owing to the difficulty in deciding when to resume treatment in the Ex-PRESS group.
The control of filtering bleb formation is an important aspect of care post-filtering surgery. Our results suggest that the method, timing, and effect of postoperative treatment might differ between groups. Further studies on postoperative treatment after Ex-PRESS insertion are necessary. Qualitative and quantitative analyses of optical coherence tomography in the filtering bleb15,16 might allow the differentiation of bleb formation of between groups.
Regarding postoperative complications, a previous meta-analysis reported a significantly lower incidence of hyphema in Ex-PRESS groups, but no intergroup differences in other complications.8 In our present study, the incidence of hyphema was significantly lower in the Ex-PRESS group. Ex-PRESS insertion requires neither sclerotomy nor peripheral iridectomy, and probably causes less hyphema or inflammation. We therefore recommend Ex-PRESS insertion in patients treated with oral anticoagulants due to the high risk of postoperative hyphema and in patients with neovascular glaucoma. In our patients with neovascular glaucoma, the level of IOP decline at 6 months after surgery was comparable between the two groups, and we saw no significant difference between patients with neovascular glaucoma and those with primary open-angle glaucoma. However, because neovascular glaucoma is refractory, we do not know if comparable outcomes should be expected over the long term, and further observation is necessary.
Contact between the Ex-PRESS device and iris is a specific complication of Ex-PRESS insertion. This complication was seen in eight eyes (38.1%), in which it caused the onset of a shallow anterior chamber. The depth and volume of the anterior chamber may be reduced during the early stage after Ex-PRESS insertion.17 We consider that even though the size of the drainage line can be kept constant, Ex-PRESS insertion may cause excessive filtration. The adverse impact of contact with the iris in these eight eyes was lessened with the improvement in the shallow anterior chamber. Perforation of Descemet’s membrane has also been reported.18 The direction of Ex-PRESS insertion and number of sutures in the scleral flap require further investigation.
Ex-PRESS insertion in the present study was done using MMC. No MMC-associated complications were seen. However, even though Ex-PRESS insertion is technically easier than TLE, it is unlikely that Ex-PRESS insertion with MMC is superior to TLE in terms of the incidence of postoperative complications, including endophthalmitis.
Several limitations of our study warrant mention. First, the number of patients was small and the observation period was short. Second, filtering bleb formation was visually assessed by measurement of the height of the filtering bleb only, and neither visual acuity, visual field, iritis, ocular surface damage, pachymetry, nor corneal endothelial cells was investigated before or after surgery. We are now planning a longer-term study in a larger number of patients, and a qualitative and quantitative analysis of optical coherence tomography in the filtering bleb.
Disclosure
The authors report no conflicts of interest in this work.
References
Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG. Glaucoma Volume 2: Surgical Management. Philadelphia, PA, USA: Saunders Elsevier; 2009:546–562. | ||
Dahan E, Carmichael TR. Implantation of a miniature glaucoma device under a scleral flap. J Glaucoma. 2005;14(2):98–102. | ||
Maris PJ Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma. 2007;16(1):14–19. | ||
Beltran-Agullo L, Trope GE, Jin Y, Wagschal LD, Jinapriya D, Buys YM. Comparison of visual recovery following EX-PRESS versus trabeculectomy: results of a prospective randomized controlled trial. J Glaucoma. 2015;24(3):181–186. | ||
de Jong L, Lafuma A, Aguadé AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527–533. | ||
Sugiyama T, Shibata M, Kojima S, Ueki M, Ikeda T. The first report on intermediate-term outcome of Ex-PRESS glaucoma filtration device implanted under scleral flap in Japanese patients. Clin Ophthalmol. 2011;5:1063–1066. | ||
Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: a prospective, randomised study. Eye. 2012;26(5):703–710. | ||
Chen G, Li W, Jiang F, Mao S, Tong Y. EX-PRESS implantation versus trabeculectomy in open-angle glaucoma: a meta-analysis of randomized controlled clinical trials. PLoS One. 2014;9(1):e86045. | ||
Mariotti C, Dahan E, Nicolai M, Levitz L, Bouee S. Long-term outcomes and risk factors for failure with the EX-PRESS glaucoma drainage device. Eye. 2014;28(1):1–8. | ||
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. | ||
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. | ||
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. | ||
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. | ||
Good TJ, Kahook MY. Assessment of bleb morphologic features and postoperative outcomes after Ex-PRESS drainage device implantation versus trabeculectomy. Am J Ophthalmol. 2011;151(3):507–513. | ||
Singh M, Chew PT, Friedman DS, et al. Imaging of trabeculectomy blebs using anterior segment optical coherence tomography. Ophthalmology. 2007;114(1):47–53. | ||
Napoli PE, Zucca I, Fossarello M. Qualitative and quantitative analysis of filtering blebs with optical coherence tomography. Can J Ophthalmol. 2014;49(2):210–216. | ||
Hammel N, Lusky M, Kaiserman I, Robinson A, Bahar I. Changes in anterior segment parameters after insertion of Ex-PRESS miniature glaucoma implant. J Glaucoma. 2013;22(7):565–568. | ||
Tamaki R, Zako M. Interference of Descemet’s membrane with aqueous humor drainage via an ExPRESS mini shunt. Case Rep Ophthalmol. 2014;5(3):343–346. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.