Back to Journals » Breast Cancer: Targets and Therapy » Volume 7
Importance of perforating vessels in nipple-sparing mastectomy: an anatomical description
Authors Amanti C, Vitale V, Lombardi A , Maggi S , Bersigotti L, Lazzarin G, Nuccetelli E, Romano C , Campanella L, Cristiano L, Bartoloni A, Argento G
Received 4 December 2014
Accepted for publication 20 January 2015
Published 14 July 2015 Volume 2015:7 Pages 179—181
DOI https://doi.org/10.2147/BCTT.S78705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Claudio Amanti,1 Valeria Vitale,1 Augusto Lombardi,1 Stefano Maggi,1 Laura Bersigotti,1 Gianni Lazzarin,1 Emiliano Nuccetelli,1 Camilla Romano,1 Laura Campanella,1 Lara Cristiano,2 Alessandra Bartoloni,2 Giuseppe Argento2
1Breast Surgery Unit, 2Radiology Unit, University of Roma, La Sapienza Sant'Andrea Hospital, Rome, Italy
Background: Nipple-sparing mastectomy (NSM), understood as an oncologically valid procedure, is relatively new, and is an evolution of traditional mastectomy, particularly in relation to breast-conserving surgery. The anterior perforating branches are responsible for the cutaneous vascularization of the breast skin, and their preservation is a fundamental step to avoid possible postoperative necrosis. Therefore, evaluating the potential complications of cancer-related reconstructive surgical procedures such as NSM, both the distance of the tumoral lesion from the skin and the surgical incision site should be carefully considered. The preferred site of incision corresponds to the inframammary fold or possibly the periareolar area.
Methods: We retrospectively reviewed 113 patients who underwent NSM from January 2005 to October 2012 to evaluate skin complications. The anatomical study was performed by magnetic resonance imaging of the breast.
Results: Only one of the 113 women who had undergone a NSM procedure had total necrosis (0.9%) and six patients had partial necrosis (5.8%) of the nipple-areola complex.
Keywords: nipple-sparing mastectomy, breast, magnetic resonance imaging, breast perforating vessels, breast anatomy
Introduction
Nipple-sparing mastectomy (NSM) can be considered as an evolution of traditional mastectomy and represents an oncologically safe procedure, according to the principles of breast conserving surgery.
This technique preserves the entire skin envelope and the nipple-areola complex (NAC), allowing the natural breast shape to be maintained, even after a surgical procedure, in addition to obtaining a better breast reconstruction.1,2 NSM has several indisputable advantages from both an esthetic and a psychological point of view, improving the patient’s quality of life and decreasing the feeling of mutilation experienced by women undergoing traditional mastectomy.3 In order to reduce the risk of retroareolar recurrence, intraoperative irradiation of the NAC can be performed.4
The decision to select a specific incision site depends on several considerations strictly related to the arterial distribution of blood to the mammary gland. As a matter of fact, the elements of surgical anatomy play a decisive role in performing an NSM procedure, when compared with traditional mastectomy. Surgical incision lines along the medial side of the breast are associated with an increased incidence of necrotic events. However, an excessive medial extension of parenchymal dissection may interrupt the blood supply to the skin flaps, and preservation of breast skin vascularization is a prerequisite to reduce the risk of necrosis. One of the main parameters that guides the surgical approach to NSM is the tumor-nipple distance, although a preferred type of incision is not yet standardized.
Methods
The anterior perforating branches of the internal thoracic artery are responsible for the cutaneous vascularization of breast skin. The internal thoracic artery arises from the first portion of the subclavian artery, descends behind the lateral margin of the breast bone, externally to the pleural parietal layer, and ends approximately at the sixth intercostal space; at this level, it splits into two terminal branches, ie, the superior epigastric artery and the musculophrenic artery.
We can identify three perforating branches among the collateral arteries of the internal thoracic artery, ie, the pericardiophrenic artery, the anterior mediastinal artery, and the sternal and anterior intercostal branches. The superior perforating branch emerges from the pectoralis major muscle at the second to third intercostal spaces, an intermediate one, known as “major”, at the level of the third to fourth intercostal spaces, while the lower branch emerges in correspondence with the fifth to sixth intercostal spaces. These perforating vessels supply blood to the pectoralis major muscle, to the breast skin envelope, and to the mammary gland.
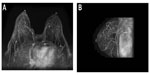
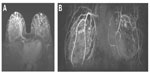
The vascular pedicle of the Major perforator is composed of: a perforating artery (as shown in Figure 1A), which bifurcates at the parasternal line into a cutaneous ventral branch and a dorsal branch directed to the mammary gland; the anterior cutaneous branch of the intercostal nerve (Figure 2B); and a perforating vein, which is a tributary of the internal thoracic vein.5
From anteroposterior projections of the chest wall (Figures 1 and 2), we can recognize: the internal thoracic artery, which runs along the lateral side of the sternum at 1.5–2 cm from the chondrosternal joint; the sternal branches, originating from the internal thoracic artery, that head first toward the sternum and, after bending medially, end as terminal vessels; and the perforating branches, which emerge from the internal thoracic artery and are ventrally directed for a few millimeters before bending medially and branching off into two ramifications, ie, a ventral cutaneous branch and a dorsal glandular branch.
Therefore, there exists an extensive network of anastomoses between the lateral thoracic artery and the vessels that originate from the internal mammary (thoracic) artery.
All our preoperative breast examinations are performed with magnetic resonance imaging as part of the internal protocol, employing a dedicated breast coil at 1.5 T magnetic field intensity (Sonata, Siemens, Washington, DC, USA). The imaging protocol includes turbo spin echo (TSE) T2-weighted axial scans, TSE T1-weighted axial images, sagittal TSE T2-weighted images, T2-weighted axial images, inversion recovery fat-suppressed TIRM T2-weighted axial images under basic conditions (TIRM with both fat and water suppression have been used in presence of silicone implants).
The study is completed with three-dimensional fast low-angle shot T1-weighted fat-saturated sequences with a sequential dynamic study during and after intravenous administration of a paramagnetic contrast agent at a dose of 0.1 mmol/kg, with standard infusion of contrast equal to 2 mL per second.
After magnetic resonance imaging of the breast, post-processing subtraction images of the gadolinium-enhanced scans are performed, as well as for the angiographic three-dimensional maximum intensity projection reconstructions oriented on coronal, axial, and sagittal views, suitable for displaying the main arterial branches and veins within the breast and their branching from internal thoracic arteries.
Another important observation concerns “wise pattern” and “modified wise pattern” mastectomies.6,7 In fact, some authors consider these procedures in the same way as NSM and, also in this case, we can assume the same anatomical considerations as outlined earlier.
The Sant’Andrea Hospital has approved the use of data for this study.
Results
We examined a subset of 113 women who had undergone a NSM procedure from January 2005 to October 2012. All the patients are diagnosed with breast cancer (51% had multifocal lesions) and the median age was 44 (range: 9–72) years. The literature data referring to NSM-related necrosis events are extremely variable, ranging from 2% to 20% and the complete loss of NAC due to necrosis has an incidence in the range of 0%–8%, with an average value of 3%–4%.8,9 The results of our analysis are quite comforting, with total necrosis of the NAC occurring in 0.9% of cases (1/103) and partial necrosis of the NAC in 5.8% (6/103).
Conclusion
Therefore, in light of our findings, we can underline the importance of the choice of incision site in avoiding post-surgical complications after breast reconstructive surgery, particularly with regard to necrosis of the NAC. To this end, the inframammary fold incision is to be preferred.
Disclosure
The authors report no conflicts of interest in this work.
References
Rusby JE, Smith BL, Gui GPH. Nipple-sparing mastectomy. Br J Surg. 2010;97:305–316. | |
Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW. Nipple-sparing mastectomy – technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68:446–450. | |
Veronesi U, Stafyla V, Luini A, Veronesi P. Breast cancer: from “maximum tolerable” to “minimum effective” treatment. Front Oncol. 2012; 2:125. | |
Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years’ experience at the European institute of Oncology of Milan (EIO). Breast Cancer Res Treat. 2009;117:333–338. | |
Chand M, Swan MC, Horlock N, Royle G. Preservation of the lateral thoracic vein in axillary dissection – its role in breast reconstruction using the DIEP flap. Breast. 2009;18:69–70. | |
Santanelli F, Paolini G, Campanale A, Longo B, Adventure C. Modified wise pattern reduction mammoplasty. A new tool for upper quadrantectomies: a preliminary report. Ann Surg Oncol. 2009;16:1122–1127. | |
Clough KB, Ihrai T, Oden S, Kaufman G, Massey E, Nos C. Oncoplastic surgery for breast cancer based on tumor location and a quadrant-per-quadrant atlas. Br J Surg. 2012;99:1389–1395. | |
Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy – complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. | |
Gieni M, Avram R, Dickson L, et al. Local breast cancer recurrence after mastectomy and immediate breast reconstruction for invasive cancer: a meta-analysis. Breast. 2012;21:230–236. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.