Back to Journals » Research and Reports in Urology » Volume 6
IL-8 secretion in primary cultures of prostate cells is associated with prostate cancer aggressiveness
Authors Neveu B, Moreel X, Deschênes-Rompré M, Bergeron A, LaRue H, Ayari C, Fradet Y, Fradet V
Received 3 December 2013
Accepted for publication 3 February 2014
Published 9 May 2014 Volume 2014:6 Pages 27—34
DOI https://doi.org/10.2147/RRU.S58643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Bertrand Neveu*, Xavier Moreel*, Marie-Pier Deschênes-Rompré, Alain Bergeron, Hélène LaRue, Cherifa Ayari, Yves Fradet, Vincent Fradet
Department of Surgery, Laval University Cancer Research Centre, CHU de Quebec Research Centre, Quebec, QC, Canada
*These authors contributed equally to this work
Background: Chronic inflammation is believed to be a major factor in prostate cancer initiation and promotion and has been studied using prostate cancer cells and immortalized cell lines. However, little is known about the contribution of normal cells to the prostatic microenvironment and inflammation. We aim to study the contribution of normal prostate epithelial cells to prostate inflammation and to link the inflammatory status of normal cells to prostate cancer aggressiveness.
Materials and methods: Short-term primary cell cultures of normal epithelial prostate cells were derived from prostate biopsies from 25 men undergoing radical prostatectomy, cystoprostatectomy, or organ donation. Cells were treated with polyinosinic:polycytidylic acid, a mimic of double-stranded viral RNA and a potent inducer of the inflammatory response. Secretion of interleukin (IL)-8 in the cell culture medium by untreated and treated cells was measured and we determined the association between IL-8 levels in these primary cell cultures and prostate cancer characteristics. The Fligner–Policello test was used to compare the groups.
Results: Baseline and induced IL-8 secretion were highly variable between cultured cells from different patients. This variation was not related to drug use, past medical history, age, or preoperative prostate-specific antigen value. Nonetheless, an elevated secretion of IL-8 from normal cultured epithelial cells was associated with prostate cancer aggressiveness (P=0.0005).
Conclusion: The baseline secretion of IL-8 from normal prostate epithelial cells in culture is strongly correlated with cancer aggressiveness and may drive prostate cancer carcinogenesis. A better characterization of individual prostate microenvironment may provide a basis for personalized treatment and for monitoring the effects of strategies aimed at preventing aggressive prostate cancer.
Keywords: cancer aggressiveness, inflammation, interleukin 8, primary cell culture
Introduction
Prostate cancer (PCa) is the commonest cancer affecting North American men and the second commonest cause of cancer mortality in the same population.1 With few exceptions, PCa lesions are adenocarcinomas stemming from basal epithelial cells located in the peripheral zone of the gland.2,3 Little is known, however, about the contribution of the normal prostate microenvironment to carcinogenesis.
Prostate epithelial cells are the first line of host defense against inflammatory stimuli, including pathogens; frequent challenges by these pathogens may lead to a state of chronic inflammation in the prostate epithelium. In a study on patients undergoing prostate biopsies, 20% of those presenting chronic inflammation in the prostate were diagnosed with PCa over a 5-year follow-up period. In contrast, only 6% of patients with no chronic inflammatory changes were diagnosed with cancer over the same period.4 Chronic inflammation is therefore suspected to be a key player in prostate carcinogenesis.5 Moreover, chronic use of anti-inflammatory agents, such as aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), has been associated with a reduction in the risk of PCa, further pointing to a role of inflammation in PCa initiation.6,7
Studies have shown that PCa cells express several cytokines, including interleukin (IL)-6 and IL-8, which are involved in the initiation, maintenance, and promotion of inflammation.8,9 IL-8 is an important proinflammatory cytokine secreted by a wide variety of normal and cancer cells8,10 from different tissues,11,12 including in the prostate.9,13 The secretion of IL-8 by PCa cells is linked to its progression,13 is increased in androgen-independent metastatic cells,14 and correlates with the metastatic potential of cancer cells.15 Furthermore, recent findings from Maxwell et al have emphasized the importance of autocrine IL-8 secretion and signaling in PCa, in which an attenuation of IL-8 signaling decreased viability of PCa cells harboring partial or complete loss of the tumor suppressor gene PTEN, an event prevalent in PCa.16 Globally, these results suggest that IL-8 may function as a significant regulatory factor within the tumor microenvironment and may be implicated in resistance to androgen deprivation and chemotherapy, in enhanced angiogenesis, and in increased tumor growth.14 However, the contribution of IL-8 secretion by normal prostate cells to prostate carcinogenesis and aggressiveness remains unknown.
Herein, we describe the baseline IL-8 secretion status and the IL-8 secretory response to an inflammatory stimulus in primary cultures of normal human prostate epithelial cells. We then examine the relationship of IL-8 secretion to PCa aggressiveness.
Materials and methods
Prostate specimens
This study was approved by the Institutional Review Board of the CHU de Quebec Hospital, Quebec, Canada. We obtained informed consent from 29 consecutive cancer patients who were scheduled for either a prostatectomy or a cystoprostatectomy procedure. Five patients (numbers 5, 12, 13, 15, and 22) could not be included in this study for the following reasons: 1) we could not obtain prostate specimens for three patients because their prostatectomy was stopped during the surgery due to extensive invasion of the cancer; 2) we could not obtain prostate specimens from one patient because we were not informed of the rescheduled date of his prostatectomy; and 3) one patient withdrew his consent. Medical charts were comprehensively reviewed. Patients having received current and/or past radiation treatment, hormonal treatment, or chemotherapy before surgery were excluded. Normal prostate tissue was also obtained from one cadaver organ donor. Tissues were obtained from prostatectomy specimen by needle biopsies taken in the normal peripheral zone of the prostate, where previous biopsies showed absence of cancer, and distant from any palpable tumor (digital rectal exam or in the prostatectomy specimen), under direct supervision of a pathologist.
Cell culture
Unless otherwise noted, all chemicals were bought from Life Technologies (Carlsbad, CA, USA). Four to five biopsy cores per prostate were transferred into sterile cold Hank’s Balanced Salt Solution (Gibco®) and centrifuged at 600 × g for 10 minutes at room temperature. Biopsies were resuspended in complete Minimum Essential Media (Life Technologies), supplemented with 10% fetal bovine serum, 100 U/mL penicillin/streptomycin, and 2 mM L-glutamine, transferred into a 35 mm cell culture dish, and cut into approximately 8 mm pieces. Collagenase III (160 U/mL; Worthington Chemical, Lakewood, NJ, USA) was added and the suspension was incubated for 15–18 hours (37°C, 5% CO2). Cells were then dispersed by pipetting, and the cell suspension was centrifuged as described above. The cell pellet was resuspended in complete keratinocyte serum-free medium ([KSFM] supplemented with 100 U/mL penicillin/streptomycin, 0.43 ng/mL endothelial growth factor, 28 μg/mL bovine pituitary extract, and 3 ng/mL cholera toxin; Sigma-Aldrich, St Louis, MO, USA), transferred into a 75 cm2 cell culture flask, and incubated (37°C, 5% CO2) for 5 days before medium replacement. Cells were confluent within 2–3 weeks. Only normal cells will grow in these conditions, as low-calcium and serum-free media do not allow proliferation of human cancer cells.17,18
Cell characterization
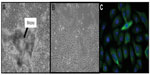
Approximately 20,000 cells were seeded per chamber on a four-chamber microscope slide. Once 60%–80% confluence was reached, cells were fixed in formaldehyde 2% for 30 minutes and washed four times in phosphate-buffered saline (PBS). Cells were permeabilized with acetone at −20°C for 3 minutes and washed three times in PBS. Then, cells were blocked in PBS/5% bovine serum albumin (BSA) for 1 hour. Primary antibodies AE1/AE3 (DAKO, Glostrup, Denmark [PBS/2% BSA (1:25)]) specific to cytokeratins (1, 2, 3, 4, 5, 6, 7, 8, 10, 13, 14, 15, 16, and 19) and vimentin (ab8978-1; Abcam Inc., Cambridge, MA, USA [PBS/2% BSA (1:50)]) were added at the indicated dilution and incubated for 2 hours at room temperature. After three washes in PBS/0.025% Tween® 20 for 5 minutes, secondary antibody Alexa Fluor 488 (Invitrogen; Life Technologies [PBS/2% BSA (1:2,000)]) was added at the recommended dilution and incubated for 1 hour in the dark. Slides were washed in PBS/0.025% Tween 20 three times for 5 minutes, after which the nuclei were stained by incubating the slides for 10 minutes in a 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) solution (Cell Signaling Technology, Danvers, MA, USA [1 μg/mL in PBS]). Cells were examined and photomicrographs were taken under fluorescent light using a Nikon A600 microscope (Nikon Instruments, Melville, NY, USA), as per the manufacturer’s instructions.
IL-8 measurements
Cells were tested after their second passage and all experiments were conducted in triplicate. Briefly, 7,200 cells were seeded per well in a 96-well plate in 50 μL of KSFM. After 24 hours, 50 μL of KSFM containing 20 μg/mL of polyinosinic:polycytidylic acid (poly(I:C); Sigma-Aldrich) was added in half of the wells to reach a final concentration of 10 μg/mL, while 50 μL of KSFM alone was added to control wells. After 20 hours, cell supernatants were collected for IL-8 measurement and total DNA was measured according to the DRAQ5® LI-COR® protocol (LI-COR Biosciences, Lincoln, NE, USA), to account for the number of cells present per well. IL-8 secretion was measured in the culture medium using the human IL-8 ELISA MAX™ Deluxe set (BioLegend, San Diego, CA, USA), as per the manufacturer’s instructions. With the exception of the time course experiment, IL-8 secretion was normalized to total DNA per well.
Data analysis
The median and interquartile range (IQR) of IL-8 expression per patient was calculated and tabulated by cancer aggressiveness. Similarly, we tabulated IL-8 secretion levels by use of NSAID, including aspirin. For statistical testing, the Fligner–Policello test19 and the Spearman’s correlations were used when the normality hypothesis could not be verified; otherwise, we used the Pearson’s chi-squared test.
We used a definition of moderately aggressive PCa as Gleason score 6 and stage less than pT2c, while aggressive PCa was defined as Gleason score 7 or greater, or stage pT2c or greater.
Results
Cohort
The study cohort was composed of 25 men, with a median age of 60.0 years (IQR: 55.0–68.0) and median preoperative prostate-specific antigen value of 4.5 ng/mL (IQR: 0.97–5.5) (Table 1). Eighteen of these men underwent a radical prostatectomy, six (in whom no clinically manifest PCa was detected before surgery) underwent a radical cystoprostatectomy for bladder cancer, and one sample was recovered from a cadaver organ donor in the context of transplant surgery. No patient had clinical evidence of chronic prostatitis, but pathological examination of the prostatectomy specimens revealed that patient 19 had evidence of chronic prostatitis. Sixteen patients had an aggressive PCa, while two patients had a moderately aggressive PCa (Table 2). There was no evidence of lymph node invasion for any patient. Pathological examination of the six cystoprostatectomy specimens showed that two patients had a moderately aggressive PCa and that four had no PCa. Bladder tumor from one patient invaded the prostate stroma (stage T4a). Pathological examination of tissues, including the prostate, from the organ donation confirmed the absence of cancer (patient 1). Complete follow-up of this cohort, over a period of up to 3 years, revealed that only one patient, recruitment patient 4, had a biochemical recurrence of PCa, with a PSA level of 0.3 ng/mL.
Primary prostate cell culture
Cells were grown for four to seven passages, each passage lasting 5–10 days, after the initial passage, which lasted 14–21 days. No significant morphological or growth kinetic differences were observed between cell cultures from the different patients. Cell characterization confirmed that the selection protocol for epithelial versus stromal cells was effective: the cultured cells expressed cytokeratins, an epithelial marker (Figure 1), but no expression of vimentin, a fibroblast marker, was observed.
Baseline and induced prostate inflammation
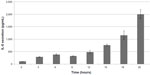
IL-8 secretion remained stable across passages 1–3. It was not affected by freeze/thaw cycles, and increased in a dose- and time-dependent manner in response to poly(I:C) (Figures 2 and 3). A plateau was not reached in these conditions even after 20 hours of incubation. Thus, based on the results shown in Figures 2 and 3, IL-8 secretion was measured after 20 hours of incubation in absence (baseline level) or presence of 10 μg/mL of poly(I:C).
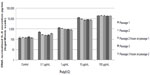
Measures of the average baseline IL-8 secretion were highly reproducible in cell cultures from a single patient, but varied considerably between cultures from different patients, ranging from 24.6–931.7 pg/mL (Figure 4). Preoperative use of NSAIDs was routinely stopped 7 days before surgery and thus did not affect baseline or induced secretion (data not shown; P=0.76 and P=0.98, respectively). Neither baseline nor induced IL-8 secretion levels were associated with age or preoperative PSA values (Pearson’s coefficient −0.007 and −0.007; P=0.74 and P=0.76, respectively; data not shown).
The induced IL-8 secretion following poly(I:C) treatment was reproducible in cell cultures from single patients, but varied considerably across cultures from different patients (range: 169.4–2,240.6 pg/mL [Figure 5]). No association was observed between baseline and induced IL-8 secretion levels (Pearson’s coefficient 0.2; P=0.34).
Prostate IL-8 secretion and cancer aggressiveness
As shown in Figure 4, the baseline IL-8 secretion level was associated with PCa aggressiveness. The median baseline IL-8 secretion levels were significantly lower in patients without PCa or having nonaggressive PCa (95.4 pg/mL, IQR: 56.5–107.9), compared to patients having aggressive PCa (309.4 pg/mL, IQR: 98.5–373.0; P=0.0005). Furthermore, we also performed Spearman’s correlation tests and found that both the Gleason score (rho =0.587, P=0.002) and the degree of aggressiveness significantly increased with the basal level of IL-8 (rho =0.63; P=0.0007). Moreover, we conducted a sensitivity analysis using two more stringent definitions of aggressive PCa: 1) either as Gleason score 7 or greater, or stage cT2c or greater, or PSA >10; or 2) Gleason score of 8 or greater, stage pT3, or PSA >20. Observed associations remained significant in these analyses, strengthening our findings (data not shown). In contrast to the results obtained with baseline IL-8 secretion, there was no association between the induced IL-8 secretion and PCa aggressiveness ([Figure 5] P=0.46).
Discussion
In this study, we used epithelial cell primary cultures derived from biopsies taken in the normal portion of prostates obtained from prostatectomies, cystoprostatectomies, or from an organ donor to study the association between IL-8 secretion and PCa aggressiveness. We used these cultures as an in vitro model reflecting the normal prostate microenvironment. We determined that baseline and induced IL-8 levels as measured in cultures of normal prostate epithelial cells varied considerably from one patient to another. We observed a strong association between baseline, but not induced, IL-8 secretion and PCa aggressiveness, whereby a higher baseline inflammatory level was associated with aggressive PCa. This suggests that the baseline inflammation level (ie, chronic resting state) of the normal prostate microenvironment may be a driver of PCa aggressiveness.
In response to infection and other proinflammatory stimuli, epithelial cells secrete chemoattractants and proinflammatory cytokines, notably IL-8.20,21 In this study, we determined that the baseline level of IL-8 secretion from normal prostate epithelial cells in culture, but not the induced level, was associated with PCa aggressiveness. This result is of critical importance since IL-8 is known to act as an early warning system and to induce the recruitment of immune and inflammatory cells20,21 that can produce oxidative molecules, thereby damaging cell components such as cell walls and DNA, potentially causing precancerous lesions. Furthermore, IL-8 is known to be a strong angiogenic factor, thus creating the perfect environment for cancer initiation and progression. Our results confirm the observations made by Murphy et al showing that IL-8 is produced by prostate epithelial cells.9 Furthermore, the association we observed between basal levels of IL-8 secretion and PCa aggressiveness agrees with the observation that levels of IL-8 secretion are higher in benign prostatic hyperplasia than in normal epithelium,22 and that levels of IL-8 secretion in the epithelium adjacent to the tumor are higher in patients who relapsed than in those who did not.23 That these observations were obtained in a model of normal epithelium cell primary culture and not in immortalized cell lines gives strength to our results and may prove useful to dissect the mechanisms leading to this observation.
Consistent with our findings, variability in the level of IL-8 and other cytokine secretion has also been observed by others in serum24,25 and in situ in a limited panel of prostate tissues.23 IL-8 secretion is regulated by many cellular processes, including transcriptional and posttranscriptional regulation and cell-signaling pathways.26,27 Deregulation of one of these cellular processes, leading to an increased secretion of IL-8, may drive carcinogenesis or cancer progression.26
IL-8 secretion was induced when primary cells were treated with the viral mimicry agent poly(I:C), in order to induce acute inflammation. The level of that induction was not associated with PCa aggressiveness. Indeed, acute inflammation is generally resolved within days and is unlikely to be associated with carcinogenesis. However, the failure to resolve acute inflammation may lead to chronic inflammation that is associated with cancer,28 as suggested by our data on baseline IL-8 secretion.
This study has some limitations. First, cell cultures from only 25 patients were used. However, these were primary cultures directly derived from well-characterized prostate biopsies from patients for whom we had a complete medical history, which is rare. Furthermore, even based on such a limited number of cell cultures, the association measured between baseline IL-8 secretion and cancer aggressiveness was strong (P=0.0005). Second, a unique cytokine (ie, IL-8) was measured in this study, based on the recognized role of this cytokine in the male genital tract, in cancer, and on the fact that IL-8 secretion appears to be an adequate surrogate marker of prostate inflammation.29,30 A more exhaustive and comprehensive analysis of the inflammatory response involving the analysis of several proinflammatory cytokines is planned as a further study. Third, epithelial prostate tissue is composed of various epithelial cell types (stem cells, definitive basal and luminal cells) at different stages of a continuum of differentiation.31 In the present study, we do not know if primary cultures of normal epithelial cells reflect the cell type composition of normal epithelial prostate tissue, but since these cells all belong to the same epithelial lineage, we do believe that they would be susceptible to the same selection criteria. Further work should include the measurement of a variety of cytokines or mediators related to inflammation as well as validation using immunohistochemistry on prostate tissue sections. Even given these limitations, the primary culture model of normal epithelial cells presented herein better reflects the in vivo state of the human prostate and the genetic diversity of men than immortalized cell lines.
Conclusion
As IL-8 may be considered a surrogate marker of prostate inflammation,29,30 our findings support the hypothesis that prostate inflammation may participate in the creation of the milieu in which aggressive PCa will develop. The dynamic measurements suggest that the level of baseline IL-8 secretion, rather than the magnitude of the induced IL-8 secretion, is likely responsible for the carcinogenesis steps leading to aggressive PCa. Thus, chronic inflammation as measured by IL-8 secretion in the prostate microenvironment may be considered a contributing factor to PCa, which is clearly a complex disease. More research to decipher the mechanisms linking baseline inflammation to early prostate carcinogenesis is needed and justified.
Acknowledgments
We are indebted to the patients who participated in this study. André Caron, PhD, was of great support in reviewing the statistical programming and data analysis. We thank Caroline Léger, PhD, for help with the writing of this article. This project was funded by grants awarded to YF (Prostate Cancer Canada Pilot Grant) and to VF (Prostate Cancer Canada Clinician Scientist Award, Prostate Cancer Canada Pilot Grant, Canadian Urological Association Scholarship Foundation, American Urological Association – Northeastern Section Young Investigator Research Grant).
Disclosure
YF is the president and chief medical officer of DiagnoCure, Quebec, QC, Canada, a life sciences company that develops and commercializes high-value cancer diagnostic tests. The authors report no other conflict of interest in this work.
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. | |
McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897–906. | |
Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329(5991):568–571. | |
MacLennan GT, Eisenberg R, Fleshman RL, et al. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J Urol. 2006;176(3):1012–1016. | |
De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. | |
Jacobs EJ, Rodriguez C, Mondul AM, et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97(13):975–980. | |
Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90(1):93–99. | |
Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. | |
Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11(11):4117–4127. | |
Remick DG. Interleukin-8. Crit Care Med. 2005;33(Suppl 12): S466–S467. | |
Standiford TJ, Kunkel SL, Basha MA, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990;86(6):1945–1953. | |
Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61(11):4569–4574. | |
Uehara H, Troncoso P, Johnston D, et al. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate. 2005;64(1):40–49. | |
Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67(14):6854–6862. | |
Aalinkeel R, Nair MP, Sufrin G, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64(15):5311–5321. | |
Maxwell PJ, Coulter J, Walker SM, et al. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur Urol. 2013;64(2):177–188. | |
Tyson DR, Inokuchi J, Tsunoda T, Lau A, Ornstein DK. Culture requirements of prostatic epithelial cell lines for acinar morphogenesis and lumen formation in vitro: role of extracellular calcium. Prostate. 2007;67(15):1601–1613. | |
Dalrymple S, Antony L, Xu Y, et al. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 2005;65(20):9269–9279. | |
Fligner MP, Policello GE II. Robust rank procedures for the Behrens-Fisher problem. J Am Stat Assoc. 1981;76(373):162–168. | |
Dooley CP, Cohen H, Fitzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321(23):1562–1566. | |
Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10. | |
Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72(1):205–213. | |
Caruso DJ, Carmack AJ, Lokeshwar VB, Duncan RC, Soloway MS, Lokeshwar BL. Osteopontin and interleukin-8 expression is independently associated with prostate cancer recurrence. Clin Cancer Res. 2008;14(13):4111–4118. | |
Hofmann JN, Yu K, Bagni RK, Lan Q, Rothman N, Purdue MP. Intra-individual variability over time in serum cytokine levels among participants in the prostate, lung, colorectal, and ovarian cancer screening Trial. Cytokine. 2011;56(2):145–148. | |
Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177(1):3–13. | |
Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7(2):122–133. | |
Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72(5):847–855. | |
Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18(26):3831–3852. | |
Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–533; discussion 533. | |
Liu L, Li Q, Han P, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009;74(2):340–344. | |
Hudson DL, Guy AT, Fry P, O’Hare MJ, Watt FM, Masters JR. Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. J Histochem Cytochem. 2001;49(2):271–278. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.