Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Highly efficient and compatible shampoo for use after hair transplant
Authors Schweiger D, Schoelermann A, Filbry A, Hamann T, Moser C, Rippke F
Received 3 April 2015
Accepted for publication 6 May 2015
Published 22 July 2015 Volume 2015:8 Pages 355—360
DOI https://doi.org/10.2147/CCID.S86015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Dorothea Schweiger,1 Andrea M Schoelermann,1 Alexander Filbry,1 Tina Hamann,1 Claudia Moser,2 Frank Rippke1
1Research and Development, Beiersdorf AG, Hamburg, Germany; 2Moser Medical, Clinics for Aesthetic Plastic Surgery, Vienna, Austria
Background: Sensitive or hyperreactive skin is a common condition defined by prickling, burning, pain, and pruritus. Although this skin problem was initially described on the face, the scalp is often affected. A sensitive scalp can react with irritation to harsh surfactants or other additives which are often present in shampoos. For this reason, we developed a new rinse-off hypertolerant shampoo specifically designed for the hypersensitive and problematic scalp.
Methods: The shampoo formulation is based on an extremely mild surfactant system and contains bisabolol, an anti-irritant and anti-inflammatory ingredient of chamomile. The shampoo is free of additives such as perfumes, silicones, colorants, parabens, paraffins, and betaine. Since skin can remain in a hyperreactive state after wounding, the status after hair transplantation was chosen as a model system to test the shampoo. Scalp condition and compatibility of each volunteer were analyzed by a plastic surgeon directly after hair transplant and after stitch removal. The plastic surgeons also rated whether they would recommend the further use of the test shampoo. Additionally, volunteers completed a self-assessment questionnaire.
Results: Following hair transplantation, regular use of the shampoo resulted in a significant reduction in the extent of scabbing and erythema. This was confirmed by dermatological scalp examinations performed by the plastic surgeon as well as in volunteers' self-assessments. The plastic surgeon highly recommended the further use of the test shampoo after hair transplant to all study participants.
Conclusion: Application of the test shampoo demonstrated excellent skin compatibility and product efficacy after hair transplant. The test shampoo significantly reduced the extent of scabs and erythema. Therefore, the shampoo is ideally suited for use after hair transplantation and for the treatment of sensitive scalp. The excellent skin compatibility is because of the mild surfactant system, the calming ingredient bisabolol, and the absence of potentially irritating ingredients.
Keywords: sensitive, scalp, shampoo, bisabolol, hair transplant
Introduction
Sensitive skin, also designated as hyperreactive skin, is a common condition.1 According to epidemiological studies performed in France,2 the UK,3 and the US,4 more than 50% of the population experience sensitive skin.
This condition is characterized by symptoms such as pricking, burning, pain, a tight sensation, and pruritus. In some subjects visible signs of inflammation are present. Environmental factors like ultraviolet radiation, heat, cold, wind, or pollution can trigger this condition as can stress and cosmetics. After wounding and injury, skin is extremely sensitive and irritable during the healing phase until regular barrier function is reestablished.5,6
Although initially described in the facial area, sensitive skin can also occur in other regions of the body, with the scalp being among the most affected.5,7 Scalp sensitivity is mainly provoked by the use of inappropriate shampoos.7 The sensitive scalp reacts with irritation to harsh surfactants or other additives such as certain perfumes or colorants which can be components of shampoos.
To successfully treat a sensitive scalp, an effective shampoo needs to comprise an extra mild surfactant system to protect the natural skin barrier and lipid system. It should also contain an agent which exerts soothing and anti-inflammatory actions. Finally, the shampoo needs to be free of potentially irritating additives.
Against this background, a special shampoo for the sensitive and problematic scalp was developed, based on an extremely mild surfactant system and containing bisabolol, an anti-irritant and anti-inflammatory ingredient of chamomile. The shampoo is free of additives such as perfumes, silicones, colorants, parabens, paraffins, and betaine.
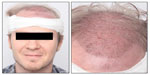
Since skin can remain hyperreactive after wounding or postoperatively,6 we selected a challenging model system to test this new shampoo formulation: the status after hair transplantation (Figure 1). Especially after hair transplant, gentle but efficient cleansing of the scalp starting 2 days after the operation is essential to prevent crusting and graft dislodgment in patients to ensure a good surgical result.8,9
In this study, we investigated the efficacy and compatibility of a rinse-off shampoo specifically developed for the sensitive scalp. Following hair transplantation, regular use of the test shampoo, starting 2 days after surgery until stitch removal, resulted in a significant reduction in the extent of scabbing and erythema. Data showed an excellent skin compatibility and product efficacy of the test shampoo, especially for the specific requirements after hair transplant. Additionally, the plastic surgeons highly recommended the further use of the test shampoo after hair transplant.
Materials and methods
Test shampoo
The test shampoo utilized in the course of the study (Eucerin® DermoCapillaire Hypertolerant Shampoo, Beiersdorf AG, Hamburg, Germany) contained decyl glucoside, sodium myreth sulfate, PEG-8, PEG-200 hydrogenated glyceryl palmate, disodium PEG-5 laurylcitrate sulfosuccinate, PEG-90 glyceryl isostearate, and bisabolol.
Application study
Forty-five subjects (42 male and three female; 20–65 years) qualified for study participation after a previous hair transplantation, which was performed at the Moser Medical, Clinics for Aesthetic and Plastic Surgery (Vienna, Austria). The aim of this study was to determine the effects of the newly developed shampoo for use in patients after hair transplantation.
In this application study, the recommendations of the current version of the Declaration of Helsinki and the guidelines of the International Conference on Harmonization Good Clinical Practice (ICH GCP) were observed as applicable to a nondrug study. All volunteers provided written, informed consent. The study was conducted in an open design under medical supervision. Medical control of the dermatological status of the study subjects was performed by trained personnel of the Moser Medical, Clinics for Aesthetic and Plastic Surgery. The study investigators monitored subjects for unexpected skin reactions and surveyed possible subjective perceptions of discomfort. Also, all subjects were recruited by the Moser Medical, Clinics for Aesthetic and Plastic Surgery.
Approximately 2 days after hair transplant, volunteers started to use the test shampoo and continued until their stitches were removed (between 7 and 23 days). They were instructed to utilize a realistic amount of shampoo and to wash their scalp at least once a day with it. During the study, subjects were required to refrain from using other shampoos as well as other scalp treatments. The test area was the scalp, in particular, the area where hair follicles were transplanted.
Determination of scalp condition and scalp compatibility by the plastic surgeon
Directly after hair transplant (t1) and also after removal of stitches (t2), the plastic surgeons carried out dermatological scalp examinations with respect to the severity of scabs and extent of erythema using the following 5-point rating scale: 0= none, 1= slight, 2= moderate, 3= substantial, 4= extreme.
Also, at stitch removal, scalp compatibility in every subject was assessed based on the following 5-point rating scale: 0= very poor; 1= poor; 2= moderate; 3= good; 4= very good. After stitch removal, the plastic surgeons were asked to answer the question: “From a dermatological point of view, would you further recommend this product to this patient?” with a “yes” or “no”.
Determination of efficacy and scalp compatibility by subjects
At the time of stitch removal, volunteers completed a self-assessment questionnaire to rate shampoo efficacy and scalp compatibility based on the following 8-point rating scale: 0= cannot be assessed, 1= does not apply at all, 2= does not apply, 3= does rather not apply, 4= neutral, 5= rather applies, 6= applies, 7= applies completely. Particularly, subjects established the agreement with the following statements: (A) “The test shampoo has a fine and creamy foam”, (B) “The test shampoo has a good cleansing performance”, (C) “The test shampoo can easily be washed out”, (D) “The test shampoo cares for my hair”, (E) “The test shampoo is suitable for use after hair transplant”, (F) “The test shampoo gently removes scabs”, (G) “The test shampoo calms the scalp”, (H) “The test shampoo alleviates itching”, (I) “The test shampoo does not have a burning sensation on the scalp”, (J) “The test shampoo is particularly mild and gentle to the scalp”, and (K) “The test shampoo is particularly skin tolerable”.
Statistical analysis
A significance level of 0.05 (α) was chosen for statistical analysis, based on two-sided hypothesis testing. For analysis, Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and the SAS software (SAS Institute Inc., Cary, NC, USA) package for Windows V9.2 were used.
The dermatological assessment regarding scabs and erythema was conducted using a comparison among points in time employing the Wilcoxon’s signed rank test. Questionnaires were evaluated using a test for relevance of the inquired statement utilizing a binomial test for null hypothesis proportion p0=0.5.
For analysis of self-assessment, absolute and relative frequencies were represented by tables and graphs based on the original data. The relevance of the inquired statements was tested using a binominal test for null hypothesis proportion p0=0.5. Due to rounding errors, the sum of the relative frequencies may deviate from 100%.
Results
Determination of scalp condition and scalp compatibility by the plastic surgeon
All 45 subjects completed the study and were considered for analysis. As observed by the plastic surgeon, no volunteer showed an incompatibility reaction.
Figure 2 illustrates that immediately after surgery, the surgeon assessed the severity of scabs as 1.67±0.64. When stitches were removed, this value was significantly decreased to 1.00±0.64 (P<0.001). Postoperatively, the extent of erythema was rated as 1.64±0.68. Following removal of stitches, this score value had significantly declined to 0.13±0.40 (P<0.001). In all subjects, the extent of scabbing as well as the extent of erythema was significantly lower during the removal of stitches than immediately after surgery.
  | Figure 2 Decrease in the extent of scabbing and erythema as determined by the plastic surgeon. |
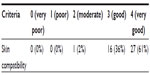
When stitches were removed, the plastic surgeon assessed the scalp compatibility of the test shampoo as good to very good (97%; Table 1). Also, based on dermatological scalp examination, the plastic surgeon recommended further use of the test shampoo after hair transplant to all study participants (100%; P<0.001).
  | Table 1 Absolute and relative (%) frequencies and mean scores of scalp compatibility assessment as determined by the plastic surgeon |
Determination of scalp condition and scalp compatibility by subjects
To assess the efficacy of the test shampoo after hair transplant, subjects completed a dermatological questionnaire at the time of stitch removal (Figure 3A and B). An answer belonging to category 0–4 was valued as “no agreement” and an answer belonging to category 5–7 was ranked as “agreement: applies”.
All statements were confirmed with statistical evidence (P<0.001). Two self-assessment questionnaires were lacking isolated values because these volunteers did not answer all questions.
In the self-assessments, the volunteers significantly confirmed the reduction of itching, a scalp-calming effect, good product mildness, and cleansing performance as well as good hair care effects after shampoo treatment.
With respect to the application of the shampoo, no incompatibility reactions were reported in volunteers’ self-assessments.
Discussion
Sensitive skin is a common condition of the scalp, and the most common symptoms associated with this condition are pruritus (approximately 60%) and prickling (approximately 30%).5,10 Misery et al10 report that the incidence of a self-declared hyperreactive scalp is increasing with increasing age. The authors speculate that this could be due to an age-related loss of nerve endings or a chronic use of irritants present in regularly applied shampoos. However, the underlying pathophysiology of sensitive skin is still unclear. A tendency to barrier impairment and an increased permeability of the stratum corneum have been discussed. Accordingly, skin hyperreactivity to water-soluble irritants could be induced by a larger quantity of irritants that penetrate the skin.1,11 Nonspecific inflammation may also be associated with the release of prostaglandin E2.12 As a treatment strategy for sensitive skin, the use of well-tolerated cosmetics or the use of cosmetics with soothing effects has been recommended.13
In this context, a new shampoo for the treatment of sensitive scalp was developed containing an extremely mild surfactant system for a gentle cleansing to avoid irritation. Additionally, skin soothing bisabolol, a sesquiterpene alcohol found in chamomile, was incorporated into the formulation.14 This active compound has been demonstrated to decrease proinflammatory cytokine production.15 Also, in experimental models of acute dermatitis, this agent exerted topical anti-inflammatory effects. Furthermore, bisabolol may be effective in the treatment of skin inflammatory disorders such as atopic or contact dermatitis as well aspsoriasis.16 Finally, the test shampoo was free of irritating additives.
Since symptoms of a hyperreactive scalp are primarily of a subjective nature, may vary, and can be triggered by a multitude of factors,8 it is not easy to establish uniform clinical conditions for the test of such shampoos. Therefore, we chose the status after hair transplant as a highly challenging model system to test this new shampoo.
All subjects underwent FUT (follicular unit transplantation) surgery. This technique is currently considered the gold standard in hair transplantation. In the course of FUT, a large section of the scalp tissue is removed for follicular unit harvest, grafts are dissected and stored, the recipient area is prepared with slits, and finally, grafts are positioned. Especially after hair transplantation, the scalp is very sensitive and needs special care to avoid graft dislodgment and to ensure a good surgical result in patients. Gentle but efficient shampooing of the scalp to prevent crusting, starting 2 days postoperation, done once per day is highly recommended.8,9
Considering these prerequisites, the new shampoo reduced scab and erythema. This correlation was proven by comparison of the dermatological ratings of the plastic surgeon directly after hair transplant and at the time of stitch removal. It is crucial to gently remove scabs since avoidance of crusting reduces the occurrence of graft dislodgment during the first 7 days after hair transplant. Also, adherent scab removal at 2–5 days after the operation may result in lost grafts.17 Although the scalp is well vascularized and, hence, serious infections are scarce, they can occur with insufficient hygiene or elevated crust formation.18 The study results indicate that the use of the test shampoo facilitates the healing process and soothes the scalp.
At stitch removal, the plastic surgeon performed a dermatological scalp examination and assessed the skin compatibility of the test shampoo as good to very good. The test shampoo was very well-tolerated by the study population over the entire duration of usage. This finding was further corroborated by the fact that the surgeon recommended the further use of the test shampoo after hair transplant to all study participants (100%).
Since symptoms of scalp sensitivity are predominantly subjective, volunteers were asked to fill out questionnaires when stitches were removed to rate various efficacies and scalp compatibility statements. Data demonstrated that subjects perceived significant benefits from the test product. They stated examples with significant agreement that the shampoo gently removes scabs and alleviates itching. Both characteristics are extremely important after hair transplantation to ensure a smooth healing process and a satisfactory outcome of the transplantation.
Conclusion
In conclusion, our data show that the application of the newly developed shampoo containing an extremely mild surfactant system and the calming ingredient bisabolol to the sensitive scalp after hair transplant results in excellent skin compatibility and product efficacy. Also, the test shampoo significantly reduces the extent of scabs and erythema. Therefore, it is ideally suited for use after hair transplant and also for general treatment of sensitive scalp.
Acknowledgments
We thank the employees of the Moser Medical, Clinics for Aesthetic and Plastic Surgery, Vienna, Austria, for conducting the study. We also thank Juliane Lüttke for statistical analyses.
Disclosure
The authors report no conflicts of interest in this work.
References
Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci. 2013;35(1):2–8. | |
Guinot C, Malvy D, Mauger E, et al. Self-reported skin sensitivity in a general adult population in France: data of the SU.VI.MAX cohort. J Eur Acad Dermatol Venereol. 2006;20(4):380–390. | |
Willis CM, Shaw S, De Lacharrière O, et al. Sensitive skin: an epidemiological study. Br J Dermatol. 2001;145(2):258–263. | |
Jourdain R, de Lacharrière O, Bastien P, Maibach HI. Ethnic variations in self-perceived sensitive skin: epidemiological survey. Contact Dermatitis. 2002;46(3):162–169. | |
Misery L, Sibaud V, Ambronati M, Macy G, Boussetta S, Taieb C. Sensitive scalp: does this condition exist? An epidemiological study. Contact Dermatitis. 2008;58(4):234–238. | |
Kottner J, Hillmann K, Fimmel S, Seité S, Blume-Peytavi U. Characterisation of epidermal regeneration in vivo: a 60-day follow-up study. J Wound Care. 2013;22(8):395–400. | |
Saint-Martory C, Roguedas-Contios AM, Sibaud V, Degouy A, Schmitt AM, Misery L. Sensitive skin is not limited to the face. Br J Dermatol. 2008;158(1):130–133. | |
Vogel JE, Jimenez F, Cole J, et al. Hair restoration surgery: the state of the art. Aesthet Surg J. 2013;33(1):128–151. | |
Bunagan MJ, Banka N, Shapiro J. Hair transplantation update: procedural techniques, innovations, and applications. Dermatol Clin. 2013;31(1):141–153. | |
Misery L, Rahhali N, Ambonati M, et al. Evaluation of sensitive scalp severity and symptomatology by using a new score. J Eur Acad Dermatol Venereol. 2011;25(11):1295–1298. | |
Seidenari S, Francomano M, Mantavoni L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38:311–315. | |
Reilly DM, Parslew R, Sharpe GR, et al. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm Venereol. 2000;80:171–174. | |
Misery L. Sensitive Skin, BASF Skin Care Forum; 2013. Available from: http://www.skin-care-forum.basf.com/de/artikel/home/new-concepts-in-acne-pathogenesis/2013/12/[email protected]/sensitive-skin/2013/03/21?id=c5507340-a11c-4146-a79a-dba915b09c18&mode=Detail. Accessed February 16, 2015. | |
Kamatou GPP, Viljoen AM. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc. 2010;87:1–7. | |
Maurya AK, Singh M, Dubey V, Srivastava S, Luqman S, Bawankule DU. α-(−)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol. 2014;15(2):173–181. | |
Leitea O, Leitea LHI, Sampaioa RS, et al. (−)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia. 2011;82(2):208–211. | |
Bernstein RM, Rassman WR. Graft anchoring in hair transplantation. Dermatol Surg. 2006;32:198–204. | |
Farjo N. Infection control and policy development in hair restoration. Hair Transplant Forum Int. 2008;18:141–144. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.