Back to Journals » The Application of Clinical Genetics » Volume 8
Hardy–Weinberg equilibrium analysis of the 48 bp VNTR in the III exon of the DRD4 gene in a sample of parents of ADHD cases
Authors Trejo S, Toscano-Flores J, Matute E , Ramírez-Dueñas MDL
Received 14 September 2014
Accepted for publication 8 December 2014
Published 2 June 2015 Volume 2015:8 Pages 133—136
DOI https://doi.org/10.2147/TACG.S74300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Salvador Trejo, José J Toscano-Flores, Esmeralda Matute, María de Lourdes Ramírez-Dueñas
Laboratorio de Neuropsicología y Neurolingüística, Instituto de Neurociencias CUCBA, Guadalajara, Jalisco, Mexico
Abstract: The aim of this study was to obtain the genotype and gene frequency from parents of children with attention-deficit/hyperactivity disorder (ADHD) and then assess the Hardy–Weinberg equilibrium of genotype frequency of the variable number tandem repeat (VNTR) III exon of the dopamine receptor D4 (DRD4) gene. The genotypes of the III exon of 48 bp VNTR repeats of the DRD4 gene were determined by polymerase chain reaction in a sample of 30 parents of ADHD cases. In the 60 chromosomes analyzed, the following frequencies of DRD4 gene polymorphisms were observed: six chromosomes (c) with two repeat alleles (r) (10%); 1c with 3r (1.5%); 36c with 4r (60%); 1c with 5r (1.5%); and 16c with 7r (27%). The genotypic distribution of the 30 parents was two parents (p) with 2r/2r (6.67%); 1p with 2r/4r (3.33%); 1p with 2r/5r (3.33%); 1p with 3r/4r (3.33%); 15p with 4r/4r (50%); 4p with 4r/7r (13.33); and 6p with 7r/7r (20%). A Hardy–Weinberg disequilibrium (χ2=13.03, P<0.01) was found due to an over-representation of the 7r/7r genotype. These results suggest that the 7r polymorphism of the DRD4 gene is associated with the ADHD condition in a Mexican population.
Keywords: ADHD, parents, DRD4, HWE
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a developmental condition characterized by inattention, hyperactivity, and impulsivity that has a prevalence of 5.29% in childhood and, in some cases, may persist into adulthood.1 ADHD is considered a multifactorial disorder because it results from the interaction of multiple genes and environmental factors, though the genes responsible and their respective contributions have not yet been clarified.2,3 Faraone et al4 estimate a heritability of 76%, which is the proportion of phenotypic variance attributable to genetic variance. The remaining 24% represents the disorder variance attributable to environmental variation, in which case a patient’s first-order relatives could have genes related to ADHD but may not report symptoms of the disorder.
Genetic studies have focused on the dopaminergic network due to two significant findings: 1) the efficacy of methylphenidate as a treatment that blocks the dopamine transporter and 2) its relation to the executive functions and the frontostriatal network.5,6 One of the most intensely studied dopamine genes is the dopamine receptor D4 (DRD4) gene, which codifies for the transmembrane protein of the type 2 dopamine receptor subgroup. The DRD4 gene is relevant to ADHD because of its expression in the anterior cingulate, an area known for its relation to such behavioral functions as attention and inhibition.7 This gene is located on the short arm of chromosome 11 (11p15.5) and consists of three introns and four exons.8 To date, a 48 bp variable number tandem repeat (VNTR) allele polymorphism with between two (DRD4-IIIe-2r) and eleven (DRD4-IIIe-11r) repeats has been identified in the III exon.9 The 48 bp VNTR of the DRD4’s III exon polymorphism shows high variability across world populations; for example, DRD4-IIIe-7r polymorphism frequencies are higher in Native Americans and lower in European populations.10
In addition, it has been pointed out that the DRD4-IIIe-7r polymorphism is associated with an inaccurate, impulsive response style on neuropsychological tasks.11 While these behaviors are characteristic of ADHD disorder, the allele frequencies of the DRD4 polymorphisms in the first-order relatives of an ADHD patient have been studied very little in Mexican populations. Therefore, the aim of this work is to establish the genotype and gene frequencies in parents of ADHD cases, and assess the Hardy–Weinberg equilibrium of genotype frequency in relation to the 48 bp VNTR III exon of the DRD4 gene.
Materials and methods
The present study was approved by the Ethics Committee of the Neuroscience Institute in Guadalajara, Mexico.
Participants
The parents of ADHD cases were recruited from a sample of children previously diagnosed with ADHD in a study of the prevalence of this disorder among Mexican school-aged children (Barrios O et al, unpublished data, 2015). With regards to subjects’ demographic data, we chose children from public schools, since they represent 92% of Mexico’s elementary school population.12 Thirty-six families of children with ADHD were contacted and 19 of them accepted the invitation to participate in the study (refusal rate =47%). Both parents participated in eleven families, in five families only the mother was available, and in three cases only the father was tested. Thus, the final sample consisted of 30 parents (14 men, 16 women) with a mean age of 49.1 years (±7.45 years). All the parents answered a questionnaire based on the ADHD criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and a retrospective Wender Utah Rating Scale (WURS) of ADHD symptoms in childhood.13 The mean they reported for symptoms of inattention on the ADHD questionnaire was 1 (±1.66), while the mean for DSM-IV hyperactive symptoms was 1.22 (±1.68). Finally, the mean on the WURS scale was 21 points (±13.66). The academic level of the parents was 10% elementary school, 20% middle school, 16.7% technical school, 6.7% high school, 43.3% bachelor’s degree, and 3.3% master’s degree. The study sample comes from Guadalajara’s mestizo population, as defined by Leal et al14 in their human leukocyte antigen study. The inclusion criteria were 1) IQ ≥80 and 2) no neurological or psychiatric disorders.
Molecular analysis
Peripheral blood samples were obtained from all parents in tubes containing ethylenediaminetetraacetic acid as an anticoagulant. Deoxyribonucleic acid (DNA) extraction was achieved using the salting-out method.15 DNA concentration and purity were determined and quantified spectrophotometrically. Amplification of the 48 bp VNTR located in the III exon of the DRD4 gene was performed using the primers (5′-AGGTGGCACGTCGCGCCAAGCTGCA-3′ sense; 5′-TCTGCGGTGGAGTCTGGGGTGGGAG-3′ antisense) under the conditions previously described by Muramatsu et al.16
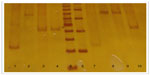
Determination of the type of allele was made by electrophoresis in polyacrylamide gels stained with silver nitrate. The size of the alleles was established by comparing the existing gel bands with commercial molecular weight markers (see Figure 1).17
Statistical analysis
The Hardy–Weinberg equilibrium was analyzed in relation to the 7r polymorphism, such that q represents the frequency of that polymorphism, while p grouped together all the polymorphisms with fewer repetitions. Finally, to compare the expected genotype frequencies against the observed frequencies, we used a chi-square test applied through the Hardy–Weinberg equilibrium calculator, in addition to a Fisher’s exact test.18
Results
The genotype distribution of the DRD4 III exon polymorphisms in the parents was two individuals (6.68%) with the 2r/2r genotype, one (3.33%) with the 2r/4r genotype, one (3.33%) with the 2r/5r genotype, one (3.33%) with the 3r/4r genotype, 15 (50%) with the 4r/4r genotype, four (13.33%) with the 4r/7r genotype, and six (20%) with the 7r/7r genotype. The gene frequency of the 60 genes was as follows: six 2r polymorphisms (10%), one 3r polymorphism (1.5%), 36 4r polymorphisms (60%), one 5r polymorphism (1.5%), and 16 7r polymorphisms (27%).
We found that the parental genotypic frequency was in Hardy–Weinberg disequilibrium (χ2=13.03, P<0.01; P=0.044, Fisher’s exact test), while the statistical difference favored a high number of 7/7 genotypes. This analysis was conducted with an expected frequency of p2=16 individuals, 2pq =12 individuals, and q2 = two individuals, but the observed frequencies were p2=20 individuals, 2pq = four individuals, and q2 = six individuals.
Discussion
In this sample of parents of ADHD cases, the allelic distribution of the DRD4 gene showed a higher frequency of the DRD4-IIIe-4r polymorphism, followed by the DRD4-IIIe-7r polymorphism. This result was expected because DRD4-IIIe-4r has been proposed as a progenitor of the DRD4 gene in humans, and the DRD4-IIIe-7r polymorphism is a mutational event with a positive selection.9 According to these results, the DRD4-IIIe-4r polymorphism could be the wild type in this sample from Guadalajara, which would mean that it is similar to other frequencies around the world (see Table 1). These results contrast with those of Chang et al,10 who argue that the DRD4-IIIe-7r polymorphism is the most prevalent one in Native Americans; but in all other regards our results are similar to reports on European populations. Thus, our findings suggest that the population of Guadalajara is the product of a mixture of different peoples, as Leal et al14 have pointed out.
  | Table 1 Proportions of the allelic frequencies of the dopamine receptor D4 gene around the world |
According to the Hardy–Weinberg model and the gene frequencies from this study, a significant difference was found between the expected and observed genotypes. This disequilibrium is related to an over-representation of DRD4-IIIe-7r polymorphism homozygosity. Although these results are from a small sample, their presence is significant when compared with both an open population study conducted in Mexico City by Martínez-Levy et al,19 who found a Hardy–Weinberg equilibrium of polymorphic variants of DRD4, and the work of Flores-Aréchiga et al,20 who demonstrated a similar gene frequency between populations in Jalisco and Mexico City. Thus, the unexpected disequilibrium found in our study suggests that the presence of DRD4-IIIe-7r is directly related to the selection of the parents of ADHD cases.
In summary, this small sample presents an over-representation of the DRD4-7r polymorphism that is an ADHD candidate gene. Considering the Hardy–Weinberg disequilibrium found in this study of individuals who are parents of children with ADHD, and the equilibrium reported in an open Mexican population by Martínez-Levy et al, we can assume that these parents share some ADHD genetic characteristics with their children, although they do not manifest ADHD.19
Conclusion
In conclusion, this study illustrates the value of considering the genetic analysis of parents of ADHD children to better understand the relationship between genetic and environmental factors in the expression of the behaviors related to this disorder. In light of these results, future studies could be conducted to further explore this important issue.
However, it is important to highlight some limitations in relation to future studies: 1) the small sample size means that we must be cautious in terms of generalizing these results, although the quantity of 60 chromosomes is not a negligible size for such genetic population studies; 2) the fact that no control group consisting of parents of typical children was included; and 3) the need for molecular genetic association studies that replicate our results.
Acknowledgments
This work was supported by Mexico’s National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología – CONACyT, Grant Number #84494). Participating investigators: Omar Barrios, Yaira Chamorro, and Lourdes Bolaños, who provided and cared for study participants.
Authors’ contributions
ST, EM, and MdLR-D contributed to the conception and design of the experimental protocol and to the analysis and interpretation of data. They also drafted and revised the paper and gave final approval for the version to be published. JT-F contributed to data acquisition, the drafting and revising of the paper, and final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition. Arlington, VA, USA. 2013. | |
Franke B, Neale B, Faraone S. Genome-wide association studies in ADHD. Hum Genet. 2009;126(1):13–50. | |
Johns Hopkins University, Baltimore, MD. MIM Number: 1434652013. | |
Faraone S, Perlis R, Doyle A, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. | |
DiMaio S, Grizenko N, Joober R. Dopamine genes and attention-deficit hyperactivity disorder: a review. J Psychiatry Neurosci. 2003;28(1):27–38. | |
Banaschewski T, Becker K, Scherag S, Franke B, Coghill D. Molecular genetics of attention-deficit/hyperactivity disorder: an overview. Eur Child Adolesc Psychiatry. 2010;19(3):237–257. | |
Kebir O, Joober R. Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies. Eur Child Adolesc Psychiatry. 2011;261(8):583–594. | |
Aguirre-Samudio AJ, Nicolini H. El gen receptor a dopamina D4 y su asociación con los trastornos mentales. Rev Invest Clin. 2005;57:65–75. | |
Ding Y-C, Chi H-C, Grady D, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci U S A. 2002;99(1):309–314. | |
Chang F-M, Kidd J, Livak K, Pakstis A, Kidd K. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98(1):91–101. | |
Langley K, Marshall L, Bree M, et al. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161(1):133–138. | |
Sistema Educativo de los Estados Unidos Mexicanos. Ciclo Escolar 2002–2003. Secretaria de Educación Pública. Available from: http://www.sep.gob.mx/work/models/sep1/Resource/1899/1/images/publicacion2003.pdf. Accessed December 12, 2014. | |
Ward M, Wender P, Reimherr F. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885–890. | |
Leal C, Mendoza-Carrera F, Rivas F, Rodriguez-Reynoso S, Portilla-de Buen E. HLA-A and HLA-B allele frequencies in a mestizo population from Guadalajara, Mexico, determined by sequence-based typing. Tissue Antigens. 2005;66(6):666–673. | |
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. | |
Muramatsu T, Higuchi S, Murayama M, Matsushita S, Hayashida M. Association between alcoholism and the dopamine D4 receptor gene. J Med Genet. 1996;33(2):13–115. | |
Vieyra G, Moraga M, Henríquez H, Aboitiz F, Rothhammer F. Distribución de alelos de los genes DRD4 y DAT1 del sistema dopaminérgico en la población mixta de Santiago de Chile. Rev Méd Chile. 2003;131:135–143. | |
Rodriguez S, Gaunt T, Day I. Hardy–Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169(4):505–514. | |
Martínez-Levy G, Benjet C, Briones-Velasco M, Pérez-Molina A, Nani A, Sabás C. Genetic variability study of DRD4 and DAT1 in the urban population of Mexico City. Salud Mental. 2013;36(3):189–192. | |
Flores-Aréchiga A, Gómez-Espinel I, Castro-Cárdenas L, Treviño-Zúñiga J, Silva-Ramirez B, Cerda-Flores R. Estructura genética de tres poblaciones mexicanas en base al sistema HLA-A. Revista de la Facultad de Salud Pública y Nutrición. 2009;10:4. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.