Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 8
Glycosylated hemoglobin as a screening test for hyperglycemia in antipsychotic-treated patients: a follow-up study
Authors Steylen P, van der Heijden F, Hoogendijk W, Verhoeven W
Received 26 June 2014
Accepted for publication 13 August 2014
Published 23 January 2015 Volume 2015:8 Pages 57—63
DOI https://doi.org/10.2147/DMSO.S70029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Pauline MJ Steylen,1 Frank MMA van der Heijden,1 Witte JG Hoogendijk,2 Willem MA Verhoeven1,2
1Vincent van Gogh Institute for Psychiatry, Center of Excellence for Neuropsychiatry, Venray, the Netherlands; 2Erasmus University Medical Center, Department of Psychiatry, Rotterdam, the Netherlands
Purpose: To assess the point prevalence of undetected prediabetes (preDM) and diabetes mellitus (DM) in patients treated with antipsychotics and to compare metabolic parameters between patients with normoglycemia (NG), preDM, and DM. Furthermore, conversion rates for preDM and DM were determined in a 1-year follow-up.
Patients and methods: In a naturalistic cohort of 169 patients, fasting glucose (FG) and hemoglobin A1c (HbA1c) criteria were applied at baseline and at follow-up after 1 year. A distinction was made between baseline patients diagnosed according to FG (B-FG) and those diagnosed according to HbA1c (B-HbA1c). Conversion rates in the 1-year follow-up were compared between B-FG and B-HbA1c.
Results: At baseline, preDM and DM were present in 39% and 8%, respectively. As compared to patients with NG, metabolic syndrome was significantly more prevalent in patients with preDM (62% vs 31%). Although the majority of patients were identified by the FG criterion, HbA1c contributed significantly, especially to the number of patients diagnosed with preDM (32%). Regarding the patients with preDM, conversion rates to NG were much higher in the B-FG group than in the B-HbA1c group (72% vs 18%). In patients diagnosed with DM, conversion rates were found for B-FG only.
Conclusion: PreDM and DM are highly prevalent in psychiatric patients treated with antipsychotic drugs. HbA1c was shown to be a more stable parameter in identifying psychiatric patients with (an increased risk for) DM, and it should therefore be included in future screening instruments.
Keywords: severe mental illness, prediabetes, diabetes, fasting glucose, HbA1c
Introduction
Diabetes mellitus (DM) is a chronic illness that has been associated with a two- to three-fold increased incidence of cardiovascular disease independent of other well-known risk factors.1,2 For decades, either the fasting glucose (FG) or the 2-hour value in the oral glucose tolerance test was used as a major criterion for the diagnosis of DM. Since glycosylated hemoglobin A1c (HbA1c) is a reliable measure of long-term glycemic exposure and is strongly correlated with diabetic complications, the American Diabetes Association (ADA) included HbA1c, for which no fasting conditions are necessary, as an additional diagnostic test.3,4 Hyperglycemia that does not fulfill diagnostic criteria for DM is known as prediabetes (preDM). PreDM is commonly associated with (components of) metabolic syndrome (MetS),5,6 and is a strong risk factor for the future development of DM and cardiovascular events.7,8 Detection of preDM is of special importance to prevent or delay the development of DM by means of lifestyle interventions such as dietary change and regular physical exercise.9,10 In an effort to identify persons with preDM, diagnostic categories of impaired fasting glucose, impaired glucose tolerance, and HbA1c have been established.4
Patients with severe mental illness (SMI) have a disease-related increased risk of both hyperglycemia and DM,11–13 which can further be raised by treatment with antipsychotics, known to induce or worsen glycemic dysregulation.14–16 There seems to be, however, a lack of monitoring and management of glycemic abnormalities in patients with SMI.17 Therefore, the treating clinician should be aware of the potential cardiometabolic risk, and a multidisciplinary assessment of psychiatric and somatic health care is warranted.18
In a recent study, preDM was found to be present in 37% of patients treated with antipsychotics and appeared to be correlated with markers of central obesity, dyslipidemia, and insulin resistance.19 Furthermore, it was shown that the simultaneous use of the FG and HbA1c criteria identifies the majority of patients with preDM.
The primary aim of the present study was to assess the point prevalence of undetected preDM and DM in patients treated with antipsychotics using the FG and HbA1c criteria, and to compare metabolic parameters between patients with normoglycemia (NG), preDM, and DM. In addition, conversion rates from NG to preDM and DM and vice versa were calculated in a 1-year follow-up to determine the number of patients developing or reversing glycemic abnormalities. To assess stability over time of the diagnostic parameters FG and HbA1c, baseline values of FG (B-FG) and HbA1c (B-HbA1c) were compared with those after 1-year follow-up.
Material and methods
Study design and subjects
This naturalistic cohort study was conducted at the outpatient departments of the Vincent van Gogh Institute for Psychiatry in the Netherlands and approved by the Institutional Review Board of the psychiatric training hospital. Informed consent was obtained from all patients. A health monitor was introduced as a screening instrument in a treatment and recovery program at the outpatient departments. From November 2008 to July 2011, 169 patients had complete assessments at baseline and after 1-year follow-up.
Assessment of parameters
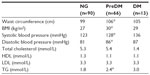
Trained nurse practitioners conducted a standardized patient interview and studied medical records to collect information about DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) diagnoses, smoking status, substance abuse, prescription of psychiatric and somatic medications, (family) history of cardiovascular disease, and/or DM. Furthermore, a physical examination was performed, including measurements of height (cm), body weight (kg), waist circumference (cm), and arterial blood pressure (mmHg). Laboratory tests comprised FG, HbA1c, triglycerides (TG), total cholesterol, high density lipoprotein (HDL) cholesterol, and low density lipoprotein cholesterol.
All patients and/or their caregivers were clearly instructed that blood sampling had to be performed under fasting conditions.
Evaluation of somatic parameters
Patients were categorized according to the FG and HbA1c criteria from the ADA as having NG (FG <5.6 mmol/L or HbA1c <5.7%), preDM (FG 5.6–6.9 mmol/L or HbA1c 5.7%–6.4%), or DM (FG ≥7.0 mmol/L or HbA1c ≥6.5%).4 Body mass index (BMI) was calculated (kg/m2), and obesity was present when BMI ≥30 kg/m2.
According to the European guidelines on cardiovascular disease prevention in clinical practice,20 a diagnosis of hypertension was based on a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication. In addition, high TG (≥1.7 mmol/L), low HDL cholesterol (female <1.3 mmol/L, male <1.0 mmol/L), and high low-density lipoprotein cholesterol (≥3.0 mmol/L or treatment with lipid-lowering agents) were defined.
MetS was defined according to the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel-III) (ATP-III) guidelines,21 and the diagnosis was made when patients fulfilled three or more of the following five criteria: waist circumference >88 cm in women and >102 cm in men, fasting blood glucose ≥6.1 mmol/L or treatment with antidiabetic medication, HDL <1.3 mmol/L in women and <1.0 mmol/L in men, TG ≥1.7 mmol/L, and blood pressure ≥130/85 mmHg or treatment with antihypertensives.
Statistical analyses
The percentages of patients who fulfilled criteria for NG, preDM, and DM according to FG or HbA1c criteria were calculated at baseline and follow-up. Independent sample t-tests and chi-square tests were used to compare demographic, clinical, and cardiometabolic parameters between patients with NG, preDM, and DM. The percentages of patients developing or reversing preDM or DM were calculated. All data were analyzed using SPSS 16.0 for Windows. Statistical significance was considered at the level of P<0.05.
Results
Demographic and clinical characteristics
In a period of 32 months, 169 patients with SMI, previously known to be nondiabetic, were examined at baseline and after 1-year follow-up. Demographic and clinical characteristics of this group are presented in Table 1. Concerning antipsychotic treatment at baseline, the majority of patients used antipsychotic monotherapy (n=148; 88%). Conventional antipsychotics were prescribed to 41 patients (28%) (haloperidol: n=18; zuclopenthixole: n=11; pimozide: n=3; flupenthixol: n=4; bromperidol: n=2; penfluridol: n=2; fluspirilene: n=1). A total of 107 patients (72%) received atypical antipsychotics (risperidon: n=42; olanzapine: n=17; quetiapine: n=10; aripiprazole: n=5; clozapine: n=32; sertindole: n=1). Twenty-one patients (12%) were treated with two antipsychotics (conventional only: n=3; atypical only: n=7; conventional and atypical: n=11). At follow-up after 1 year, there were no significant changes in antipsychotic treatment regiments or cardiometabolic parameters.
Prevalence of NG, preDM, and DM at baseline and follow-up
At baseline, 90 out of 169 patients (53%) were identified with NG, whereas 66 (39%) fulfilled criteria for preDM (FG only: n=32, HbA1c only: n=21, both: n=13) (Figure 1), and 13 (8%) were diagnosed with DM (FG only: n=11, HbA1c only: n=1, both: n=1). As compared to patients with NG, prediabetic patients had a larger waist circumference (106 cm vs 99 cm, P<0.001), higher BMI (30 kg/m2 vs 27 kg/m2, P<0.001), higher systolic blood pressure (128 mmHg vs 123 mmHg, P=0.011), higher diastolic blood pressure (86 mmHg vs 81 mmHg, P<0.001), and higher TG levels (2.4 mmol/L vs 1.8 mmol/L, P<0.001) (Table 2). As shown in Figure 2, patients with preDM were more frequently diagnosed with obesity (41% vs 20%, P=0.004), high TG (62% vs 43%, P=0.02), low HDL (58% vs 34%, P=0.004), and MetS (62% vs 31%, P<0.001). No significant differences in age, sex, family history of cardiovascular disease or DM, psychiatric diagnoses, smoking status, number or type of antipsychotic agent(s), or co-medication with antidepressants and/or mood stabilizers were found.
In comparison to diabetic patients, patients with preDM were younger (42 years vs 50 years, P=0.001). There were no significant differences in sex, family history of cardiovascular disease or DM, cardiometabolic parameters, smoking status, psychiatric diagnoses, and psychotropic treatment variables between prediabetic and diabetic patients.
After 1 year, 49 out of 169 patients (29%) were diagnosed with preDM (FG only: n=25, HbA1c only: n=16, both: n=8) and 14 (8%) with DM (FG only: n=11, HbA1c only: n=1, both: n=2). The remaining 106 patients (63%) did not fulfill the criteria for hyperglycemia and were classified as NG.
Follow-up data of patients with NG at baseline (n=90)
Of the 90 normoglycemic patients at baseline, after 1 year, 74 patients (82%) were still normoglycemic. In 13 patients (15%), conversion to preDM was found (FG only: n=11, FG and HbA1c: n=2), whereas three (3%) had developed DM (FG only: n=3).
Follow-up data of patients with preDM at baseline (n=66)
At baseline, 32 patients were diagnosed with preDM according to the FG criterion only (B-FG). As depicted in Figure 3A, after 1 year, 23 patients (72%) reversed to NG, eight patients (25%) still fulfilled criteria for preDM (FG only: n=6, FG and HbA1c only: n=2), and only one patient (3%) developed DM (FG only).
Of the 34 B-HbA1c patients with preDM, six patients (18%) reversed to NG, 24 (70%) were still diagnosed with preDM (FG only: n=5, HbA1c only: n=13, both: n=6), and four (12%) fulfilled criteria for DM (FG only: n=3, HbA1c only: n=1) (Figure 3B).
Thus, after 1 year, 28% (25% + 3%) of the B-FG patients vs 82% (70% + 12%) of the B-HbA1c patients remained in a hyperglycemic state.
Follow-up data of patients with DM at baseline (n=13)
After 1 year, three of the eleven B-FG patients (28%) reversed to NG, four patients (36%) fulfilled criteria for preDM (FG only: n=3, HbA1c only: n=1), and four patients (36%) still fulfilled criteria for DM (FG only: n=4). The two B-HbA1c patients still fulfilled criteria for DM at follow-up.
Discussion
The present study demonstrates that, using FG and HbA1c criteria, undetected preDM and DM were present in 39% and 8% of SMI patients treated with antipsychotics, respectively. Although in the present study the majority of prediabetic and diabetic patients were diagnosed by the FG criterion, HbA1c appeared to contribute significantly to the number of patients identified, especially those with preDM (32%). As compared to patients with NG, cardiometabolic parameters, including MetS, were more prevalent in patients with preDM. These findings are in line with data reported elsewhere.19
After 1 year, the prevalence of preDM had decreased about 10% but that of DM did not change. A more detailed analysis of the conversion rates, however, disclosed that over a period of 1 year, 15% vs 3% of the baseline normoglycemic patients developed preDM and DM, respectively. Of the patients with preDM at baseline, in 7% (five out of 66) DM was diagnosed at follow-up. The incidence rate of DM in these SMI patients is about ten-fold as high as compared to the general population in the Netherlands (0.2%–0.3%).22 These findings stress the high cardiometabolic risk of patients with SMI. On the other hand, it cannot be ruled out a fortiori that during this 1-year period, changes in lifestyle have contributed to a change in glucose state.
It should be underlined, however, that a substantial number of patients with a baseline diagnosis of preDM or DM showed conversion to NG at follow-up, ie, 44% or 23%, respectively. This phenomenon may be explained by the dynamic course of glucose metabolism, which stresses the need for systematic and repeated evaluation of glucose parameters.23 The reversion into a normoglycemic state also raises the question about the use of FG as a diagnostic test. In this study, it was shown that the conversion rates were higher in patients with hyperglycemia according to FG in comparison to HbA1c. After 1 year, 82% of the B-HbA1c patients remained in a hyperglycemic state as compared to only 28% of the B-FG patients. Therefore, HbA1c seems to be a more stable parameter over time in diagnosing glucose abnormalities in patients with SMI. Nevertheless, in contrast to the ADA, the World Health Organization states that there is currently insufficient evidence to make a formal recommendation on the interpretation of HbA1c levels below 6.5%.24 Furthermore, it is worth mentioning that, since HbA1c reflects the average glucose levels over the past 6–8 weeks, acute changes in glucose metabolism potentially induced by antipsychotics cannot be detected.
It is worth noting that a diagnosis of preDM or diabetes was established according to only one laboratory assessment. When glycemic abnormalities were found, patients were sent to their general practitioner for further analysis, lifestyle advice, and somatic treatment when indicated. Unfortunately, information about the number of patients in whom lifestyle modification took place was not collected and therefore its possible contribution to change in glucose metabolism could not be assessed. Another limitation is the relatively small sample size, so the results may not be representative of all antipsychotic-treated patients. Long-term follow-up studies of glucose metabolism in larger sample sizes are warranted to better understand glucose metabolism in patients with SMI.
In conclusion, systematic evaluation of glucose parameters in antipsychotic-treated patients is necessary, in order to detect glucose dysregulations at an early stage and to prevent premature metabolic and cardiovascular complications. As there is no need for a fasting condition and HbA1c is more stable over time, this test should be used, in addition to FG, to screen for glucose abnormalities in psychiatric patients at risk for DM. Further studies are warranted to assess the course of glucose dysregulation in patients with SMI in order to establish an appropriate screening instrument and somatic treatment protocol.
Acknowledgments
The authors are indebted to the patients, their caregivers, and the nursing staff for their willingness to participate in the study. All patients provided informed consent that was noticed in their medical records. Somatic screening was performed by Hans DH Kok, general practitioner at the Vincent van Gogh Institute for Psychiatry. Laboratory analyses were performed by the biochemical laboratories of the general hospital VieCuri in Venray/Venlo, the Netherlands. This study was partially supported by unrestricted grants from Astra-Zeneca, Janssen Pharmaceuticals, and Lundbeck BV.
Disclosure
The authors report no conflicts of interest in this work.
References
Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422–1426. | |
The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. | |
The International Expert Committee. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1–8. | |
American Diabetes Association. Standard of medical care in diabetes-2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. | |
Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635–643. | |
Marini MA, Succurro E, Castaldo E, et al. Cardiometabolic risk profiles and carotid atherosclerosis in individuals with prediabetes identified by fasting glucose, postchallenge glucose, and haemoglobin A1c criteria. Diabetes Care. 2012;35(5):1144–1149. | |
Petersen JL, McGuire DK. Impaired glucose tolerance and impaired fasting glucose – a review of diagnosis, clinical implications and management. Diab Vasc Dis Res. 2005;2(1):9–15. | |
Rhee SY, Woo JT. The prediabetic period: a review of clinical aspects. Diabetes Metab J. 2011;35:107–116. | |
Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. | |
Fonseca VA. Identification and treatment of prediabetes to prevent progression to type 2 diabetes. Clin Cornerstone. 2007;8(2):10–18. | |
Ryan MCM, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug naïve patients with schizophrenia. Am J Psychiatry. 2003;160(2):284–289. | |
Saddichha S, Ameen S, Akhtar S. Incidence of new onset metabolic syndrome with atypical antipsychotics in first episode schizophrenia: a six-week prospective study in Indian female patients. Schizophr Res. 2007;95(1–3):247. | |
Gladigau EL, Fazio TN, Hannam JP, Dawson LM, Jones SG. Increased cardiovascular risk in patients with severe mental illness. Intern Med J. 2014;44(1):65–69. | |
Citrome LL, Holt RI, Zachry WM, et al. Risk of treatment-emergent diabetes mellitus in patients receiving antipsychotics. Ann Pharmacother. 2007;41(10):1593–1603. | |
van Winkel R, De Hert M, Wampers M, et al. Major changes in glucose metabolism, including new-onset diabetes, within 3 months after initiation of or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorders. J Clin Psychiatry. 2008;69(3):472–479. | |
Vancampfort D, Wampers M, Mitchell AJ, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12(3):240–250. | |
Mitchell AJ, Hardy SA. Screening for metabolic risk among patients with severe mental illness and diabetes: a national comparison. Psychiatr Serv. 2013;64(10):1060–1063. | |
De Hert M, van Winkel R, Silic A, Van Eyck D, Peuskens J. Physical health management in psychiatric settings. Eur Psychiatry. 2010;25(Suppl 2):S22–S28. | |
Manu P, Correll CU, van Winkel R, Wampers M, De Hert M. Prediabetes in patients treated with antipsychotic drugs. J Clin Psychiatry. 2012;73(4):460–466. | |
Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28(19):2375–2414. | |
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. | |
Nationaal Kompas Volksgezondheid (homepage on the Internet). Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [updated June 5, 2014]. Available from: http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/endocriene-voedings-en-stofwisselingsziekten-en-immuniteitsstoornissen/diabetes-mellitus/omvang/. Accessed June 22, 2014. | |
Scheen AJ, De Hert MA. Abnormal glucose metabolism in patients treated with antipsychotics. Diabetes Metab. 2007;33(3):169–175. | |
World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation; Geneva: World Health Organization; 2011. Available from: http://www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed June 22, 2014. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.