Back to Journals » OncoTargets and Therapy » Volume 7
Gli3 silencing enhances cyclopamine suppressive effects on ovarian cancer
Received 10 November 2013
Accepted for publication 24 January 2014
Published 30 October 2014 Volume 2014:7 Pages 2007—2011
DOI https://doi.org/10.2147/OTT.S57346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Hai-Yan Liu, Zheng Dong
Department of Gynecology, First Affiliated Hospital of Liaoning Medical College, Jinzhou, People’s Republic of China
Abstract: Ovarian cancer is a leading gynecological malignancy associated with high mortality. Hedgehog signaling has been found to be important for cell proliferation and tumor growth for multiple cancers, including ovarian cancer. The present study showed that the drug cyclopamine, which blocks the hedgehog signaling pathway, could reduce cancer cell growth and proliferation and induce cell apoptosis. In addition, the silencing of the glioma-associated oncogene (Gli)3, a downstream component of the hedgehog signaling pathway, could further enhance the antitumor effects of cyclopamine. Our results suggest that Gli3 may act as resistance to cyclopamine’s effect on tumor growth. The combined treatment of cyclopamine application and Gli3 silencing therapy, therefore, may provide novel directions for clinical management of ovarian cancer.
Keywords: ovarian cancer, hedgehog, cyclopamine, proliferation, apoptosis, Gli3
Introduction
Ovarian cancer is a leading gynecological malignancy associated with high mortality. The management of ovarian cancer with pharmacological compounds is however still limited. Hedgehog signaling represents an important target in controlling cancer cell proliferation and progression, including that of ovarian cancer cells.1–5 For instance, the cyclopamine that blocks hedgehog signaling by antagonizing smoothened (smo) function has been employed to induce cancer cell apoptosis in different types of cancer cell lines, including those of prostate cancer, pancreatic cancer, ovarian cancer cells, and breast cancer.6–11
Downstream to smo protein, recent studies revealed the role of the glioma-associated oncogene (Gli) in the hedgehog signaling pathway.12–14 The Gli family includes several homologs such as Gli3, which has been proposed to be important in cancer development and tumor maintenance.15–17 Gli protein signaling has been recognized in the development of ovarian cancer;18 however, the exact role of Gli3 protein in hedgehog signaling in ovarian cancer proliferation and maintenance has not been fully investigated.
The present study examined the involvement of Gli3 signaling in the suppressive effects of cyclopamine on human ovarian cancer cell lines. The results suggest novel directions in targeting ovarian cancer at various molecular levels.
Materials and methods
Ethics statement
The study was approved by the Liaoning Medical College Animal Research Committee, and all experimental procedures were carried out in accordance with the guidelines of medical research in Liaoning Medical College.
Cell culture
The human ovarian SKOV3 cancer cell line was obtained from the Liaoning Medical College Cell Research Center and maintained in 10% fetal bovine serum, antibiotics, and RPMI 1640 culture medium. Cyclopamine at 20 μM was used to induce cell proliferation arrest and apoptosis as previously described.6
Cell proliferation analysis
The Premix WST-1 Cell Proliferation Assay (Takara Bio, Shiga, Japan) was employed for cell proliferation evaluation according to the manufacturer’s instructions in order to determine the cell proliferation rates. The assays were repeated three times.
Cell viability assay and apoptosis analysis
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was employed to examine cell viability, using a Millipore MTT kit (Billerica, MA, USA). The MTT assay was repeated five times.
To detect cell apoptosis in culture, the TUNEL kit (Roche, Basel, Switzerland) was used, and TUNEL-positive cells were counted using Hoechst counterstain for all the rest of the cells. The ratios of TUNEL positive to normal cells were calculated.
Small interfering RNA for Gli3
Gli3-specific small interfering RNAs (siRNAs) were obtained as previously described16 (siRNA-G1, 5′-UGA AUG GAA UGU UUC CGC GAC UGA A-3′; siRNA-G2, 5′-CCA UUG CAU AUG ACU UCC GCC UUA U-3′; 60 nM) (Shanghai Shengji Biotechnol, Shanghai, People’s Republic of China). Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA, USA) was used for cell culture transfection. The transfection was performed 48 hours prior to the experiment.
Xenograft
SKOV3 cancer cells (2 × 106) in 200 μL saline were injected into the intraperitoneal cavity of adult male nude mice 2 weeks prior to treatment to promote tumor growth. Mice were divided into four groups with 12 animals per group: control group received saline injection every 3 days, cyclopamine treatment group (25 mg/kg every 3 days), cyclopamine plus G1 siRNA group, and cyclopamine plus G2 siRNA group, also every 3 days. Cyclopamine was injected intraperitoneally while G1 and G2 were administered via the tail vein.
Mice were sacrificed at 4 weeks (6 animals in each group) and 7 weeks (6 animals in each group) after grafting of cancer cells, and tumor sizes were measured. Sections of the tumors were prepared for TUNEL staining, and the percentage of apoptotic cells was counted using a Zeiss microscope (Oberkochen, Germany).
Statistics
The data were represented as mean ± standard deviation and analyzed with SPSS 17.0 software (IBM Corporation, Armonk, NY, USA). The results between different groups were analyzed by Student’s t-test and ANOVA. P<0.05 was considered significantly different.
Results
Cyclopamine suppresses cancer cell proliferation
We found that the cyclopamine treatment significantly decreased cell proliferation after time lengths of 12, 24, and 48 hours (Figure 1).
Cyclopamine decreases cell viability and induces cell apoptosis
We further examined if cyclopamine decreased cell proliferation by the induction of cell apoptosis. Indeed, we found that accompanying the decrease in cell viability, TUNEL-positive cells (apoptotic) increased in a time-dependent manner in cell culture (Figure 2). Given the clear effects of cyclopamine at 24 hours after treatment, we selected 24 hours as the time point for mechanistic investigations in the following studies.
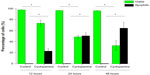  | Figure 2 Cyclopamine decreases cell viability and induces cell apoptosis at 12-, 24-, and 48-hour time points. |
Gli3 silencing further enhances cyclopamine effects
We employed two siRNAs for Gli3 (siRNA-G1 and siRNA-G2) to silence the expression of Gli3. We confirmed that the two siRNAs did reduce the expression levels of Gli3 to less than 15% in our preliminary experiments. Interestingly, this silencing of Gli3 further enhanced the cyclopamine effects to suppress the cancer cell proliferation at the 24-hour time point (Figure 3). Gli3 silencing did not affect the baseline cell proliferation rate however (Figure 3).
We further examined the usefulness of Gli3 silencing on cyclopamine effects on cell viability and apoptosis at 24 hours. Gli3 silencing did not affect the baseline cell viability or apoptosis rate, yet both G1 and G2 siRNA treatment enhanced the cyclopamine-induced cell apoptosis of cultured cells (Figure 4).
Gli3 silencing in vivo enhances the antitumor growth effect of cyclopamine
In order to further validate the effects we observed on cultured cancer cells, we performed xenograft experiments and systematically infused siRNA-G1 and siRNA-G2 via the tail vein. We found that cyclopamine treatment could effectively induce tumor size decrease (Table 1) as well as cell apoptosis in the tumor tissue (Figure 5). Both effects were enhanced with G1 and G2 treatment (Figure 5).
Discussion
Ovarian cancer is a challenging malignancy for women’s health. Recent studies highlighted the role of hedgehog signaling in ovarian cancer development and progression.18,19,21 Cyclopamine has been applied for different diseases, with proven roles in antitumor growth as well as an inducer of cell apoptosis.1,2,20,21 The present study demonstrated that Gli3 may act to exert “resistance” downstream to the hedgehog signaling pathway, thereby partly antagonizing the effects of cyclopamine. The synergistic effect of cyclopamine application and Gli3 knockdown, therefore, represents a novel strategy for clinical management of ovarian cancer.
A previous study revealed that the differences in sensitivity to cyclopamine across different pancreatic cancer cell lines were mediated by Gli3 function.16 This is in line with the current results. It will be interesting to perform gain of function studies on Gli3 to see if this could promote tumor cell growth at the baseline level. Interestingly, in our study, Gli3 silencing did not cause much change in cell proliferation, cell viability, and apoptosis compared to the baseline level. It is possible that at baseline level, Gli3 is not actively involved in tumor growth; however, Gli3 is recruited when the tumor cells were challenged with different chemotherapeutic drugs for instance.
The synergistic effects of cyclopamine and Gli3 silencing suggest that combined therapy would be more effective in targeting the hedgehog signaling pathway to manage ovarian cancer growth. There are numerous inhibitors for hedgehog signaling pathway being developed and tested in preclinical phases. Cyclopamine mainly acts on smo function.2,7 It will be important to find certain agents that target both Gli3 and smo function with a potentially high efficiency.
Other Gli3 proteins such as Gli1 have been found to be associated with ovarian cancer growth, invasion, and induced differentiation.12,22 In addition, cyclopamine or Gli1 silencing both suppressed ovarian cancer cell growth significantly.23 It will be important to investigate the potential functional redundancy between Gli1 and Gli3 in order to explore their interactions when ovarian cells are treated with cyclopamine.
Acknowledgment
The authors were supported by the Department of Gynecology at the First Affiliated Hospital of Liaoning Medical College.
Disclosure
The authors report no conflicts of interest in this work.
References
Szkandera J, Kiesslich T, Haybaeck J, Gerger A, Pichler M. Hedgehog signaling pathway in ovarian cancer. Int J Mol Sci. 2013;14(1):1179–1196. | |
Liu H, Gu D, Xie J. Clinical implications of hedgehog signaling pathway inhibitors. Chin J Cancer. 2011;30(1):13–26. | |
Chase DM, Mathur N, Tewari KS. Drug discovery in ovarian cancer. Recent Pat Anticancer Drug Discov. 2010;5(3):251–260. | |
Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29(4):469–481. | |
Liao X, Siu MK, Au CW, et al. Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through beta-catenin. Mod Pathol. 2009;22(6):839–847. | |
Che J, Zhang FZ, Zhao CQ, Hu XD, Fan SJ. Cyclopamine is a novel Hedgehog signaling inhibitor with significant anti-proliferative, anti-invasive and anti-estrogenic potency in human breast cancer cells. Oncol Lett. 2013;5(4):1417–1421. | |
Katano M. Hedgehog signaling pathway as a therapeutic target in breast cancer. Cancer Lett. 2005;227(2):99–104. | |
Sirab N, Terry S, Giton F, et al. Androgens regulate Hedgehog signalling and proliferation in androgen-dependent prostate cells. Int J Cancer. 2012;131(6):1297–1306. | |
Shaw G, Price AM, Ktori E, et al. Hedgehog signalling in androgen independent prostate cancer. Eur Urol. 2008;54(6):1333–1343. | |
Kelleher FC, McDermott R. Aberrations and therapeutics involving the developmental pathway Hedgehog in pancreatic cancer. Vitam Horm. 2012;88:355–378. | |
McCann CK, Growdon WB, Kulkarni-Datar K, et al. Inhibition of hedgehog signaling antagonizes serous ovarian cancer growth in a primary xenograft model. PLoS One. 2011;6(11):e28077. | |
Ciucci A, De Stefano I, Vellone VG, et al. Expression of the glioma-associated oncogene homolog 1 (gli1) in advanced serous ovarian cancer is associated with unfavorable overall survival. PLoS One. 2013;8(3):e60145. | |
Li H, Lui N, Cheng T, et al. Gli as a novel therapeutic target in malignant pleural mesothelioma. PLoS One. 2013;8(3):e57346. | |
Gao J, Khan AA, Shimokawa T, et al. A feedback regulation between Kindlin-2 and GLI1 in prostate cancer cells. FEBS Lett. 2013;587(6):631–638. | |
Mazumdar T, DeVecchio J, Agyeman A, Shi T, Houghton JA. The GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget. 2011;2(8):638–645. | |
Steg A, Amm HM, Novak Z, Frost AR, Johnson MR. Gli3 mediates cell survival and sensitivity to cyclopamine in pancreatic cancer. Cancer Biol Ther. 2010;10(9):893–902. | |
Katoh Y, Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol Ther. 2005;4(10):1050–1054. | |
Chen X, Horiuchi A, Kikuchi N, et al. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it’s inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007;98(1):68–76. | |
Bhattacharya R, Nicoloso M, Arvizo R, et al. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009;69(23):9090–9095. | |
Yang L, Su X, Xie J. Activation of Hedgehog pathway in gastrointestinal cancers. Vitam Horm. 2012;88:461–472. | |
Campos SM, Ghosh S. A current review of targeted therapeutics for ovarian cancer. J Oncol. 2010;2010:149362. | |
Kudo K, Gavin E, Das S, Amable L, Shevde LA, Reed E. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene. 2012;31(44):4718–4724. | |
Kandala PK, Srivastava SK. Diindolylmethane-mediated Gli1 protein suppression induces anoikis in ovarian cancer cells in vitro and blocks tumor formation ability in vivo. J Biol Chem. 2012;287(34):28745–28754. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





