Back to Archived Journals » Journal of Neurorestoratology » Volume 2
Fetal stem cells in combined treatment of chronic heart failure and their effect on morphofunctional parameters of the left ventricle myocardium and cognitive functions
Authors Klunnyk M , Sych N, Matiyashchuk I, Ivankova O, Skalozub M
Received 22 April 2014
Accepted for publication 5 June 2014
Published 30 August 2014 Volume 2014:2 Pages 107—117
DOI https://doi.org/10.2147/JN.S66612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Mariya O Klunnyk, Nataliia S Sych, Irina G Matiyashchuk, Olena V Ivankova, Marina V Skalozub
Cell Therapy Center EmCell, Kyiv, Ukraine
Aim: To investigate the effect of combined treatment with the inclusion of fetal stem cells (FSCs) on the morphology and functional dynamics of the left ventricle and cognitive functions in patients with chronic heart failure (CHF).
Materials and methods: A comparative study was carried out on patients with CHF to examine the effect of combined treatment, including the experimental application of FSCs, on the morphofunctional parameters of the left ventricle and cognitive functions. Patients were examined before FSC treatment (FSCT), and 1 month, 3 months, and 6 months after treatment. The control group consisted of 20 CHF patients of similar age, sex, and New York Heart Association class.
Results: It has been proven that FSCs positively affect objective and subjective clinical parameters. A significant reduction of serum type B brain natriuretic peptide was reported as early as 1 month after treatment. Significant increases in the left ventricle ejection fraction and decreases of the end diastolic volume were observed 6 months after treatment. Cognitive performance tests showed improvements on the Mini-Mental State Examination and Frontal Assessment Battery (conceptualization, mental flexibility, programming, sensitivity to interference, inhibitory control, and environmental autonomy) scales. The treatment resulted in significant improvements in the general score and across all cognitive areas of the Mini-Mental State Examination (recall, orientation, attention, calculation, and complex commands) after 3 months, and significant improvements across all Frontal Assessment Battery areas after 6 months. In the control group, these scores showed significant increases only at 6 months after the treatment. In the study group, depression was significantly reduced within 1 month after treatment versus 3 months in the control group. In both groups, reactive anxiety levels dropped after month 3 upon FSCT. FSCT resulted in statistically significant improvements in the contractile activity of the left ventricular myocardium, in cognitive functions, and in the emotional state of CHF patients.
Conclusion: The evidence for significant improvements in the contractile function of the left ventricle myocardium, as well as in patients' cognitive and emotional states, was observed in CHF patients after combined treatment with FSC.
Keywords: heart failure, cognitive, emotional impairment, fetal stem cells, left ventricle remodeling
Introduction
Cardiovascular diseases are among the leading causes of death worldwide and are of great concern for health care institutions in all developed countries.1,2 According to the World Health Organization, 17.3 million people died from cardiovascular diseases in 2008, representing 30% of the general mortality rate.3 The importance of this problem is different in high- and low-income countries. In the latter, 80% of people die of cardiovascular diseases, and the mortality rate is approximately the same between men and women.3 Until 2030, cardiovascular diseases – mainly cardiac illnesses and stroke as the only causes of death – are expected to take the lives of 23.6 million people each year.2,4
Chronic heart failure (CHF) is caused by the reduced contractile power of the cardiac muscle, which is insufficient for normal blood outflow from the left ventricle (LV), and which results in a low supply of oxygen and nutrients. Routine therapy is ineffective in restoring the dead cardiomyocytes.5 CHF patients usually undergo symptomatic treatment using special defibrillators, but in spite of all the medical advancements being made, the number of CHF patients is growing each year.6
Cardiovascular diseases lead to progressive damage of the cerebral tissues with marked cognitive impairment (CI) aggravating the main disease.7,8 CI is expected in CHF and leads to cognitive dysfunction, social problems, and repeated hospitalization.9,10
Commonly, CI takes place when the left ventricle ejection fraction (LVEF) is ≤30%.11 All other factors being equal (age, hypertension, and a past history of cerebrovascular diseases), the Mini-Mental State Examination (MMSE) score in elderly patients with CHF was, on an average, one unit lower than in those without CHF.3 The CI and CHF relationship is also proven by the fact that the latter is more often found among patients with cognitive dysfunction than among those without it.12,13 One of the studies showed that CHF was found in more than 20% of patients with an MMSE score <24, while in the group with better cognitive functions (CF), it occurred in <5% of cases.14 The risk of CI is very high when CHF is combined with hypertension, and when both cognitive performance in general and its elements (memory, attention, and regulation processes) are impaired.11 This points out the key role that cerebral perfusion reduction plays in CI development. This is also confirmed by the correlation between cognitive dysfunction and LVEF.15,16 In CHF, a reduction in cerebral perfusion can be combined with white matter affection (leukoencephalopathy) or atrophy of the medial temporal areas, which are very sensitive to hypoxia and hypoperfusion.17
A continuous increase in cardiovascular mortality all over the world calls for the development of new, affordable, and effective therapies for CHF complicated by CI. Fetal stem cells (FSCs), as part of a combined treatment, can open a new page in the treatment of CHF. Data from separate investigations have proven that FSCs can differentiate into cardiomyocytes, which are deficient in CHF.15,18 This results in a large increase in myocardial muscle, as well as improvements in its contractile activity and in CF.19–21
Materials and methods
Patients with New York Heart Association Functional Classification (NYHA) class III–IV CHF, diagnosed on the basis of clinical symptoms (weakness, shortness of breath, edema of the lower extremities, palpitation, and sleep apnea), and confirmed by physical examination findings, laboratory test results (serum type B brain natriuretic peptide [NT-proBNP] increase), and echocardiography findings (LVEF <45% and increased end diastolic volume [EDV] >140 mL), were selected on a voluntary basis and assigned to the main group (MG) and control group (CG). The MG consisted of 20 patients, including 14 men (70%) and six women (30%), and their ages ranged between 33 years and 60 years (mean 50.1±1.13 years). The causes of CHF included ischemic cardiomyopathy (14 patients; 70%), alcohol-induced cardiomyopathy (three patients; 15%), and dilatation cardiomyopathy (three patients; 15%). The average duration of CHF was 7.6±0.54 years and average treatment history made up 6.3±0.55 years. The number of patients with NYHA class III and class IV CHF were 16 (80%) and four (20%), respectively. The CG included 20 patients with CHF whose age and sex distributions were similar to that of the study group. In this group, CHF was caused by ischemic cardiomyopathy (number [n] =16; 80%), dilatation cardiomyopathy (n=3; 15%), and alcohol-induced cardiomyopathy (n=1; 5%). The number of patients with NYHA class III and class IV CHF was 14 (70%) and six (30%), respectively.
All patients were undergoing long-term routine CHF therapy in accordance with recommendations of the European Society of Cardiology: diuretics (torasemide; average dose: 12.5±1.3 mg), beta adrenoblockers (carvedilol; average dose: 46.9±7.9 mg), angiotensin-converting enzyme inhibitors (perindopril arginine; average dose: 6.5±0.7 mg), and/or angiotensin receptor blockers (valsartan; average dose: 100±10.5 mg); cardiac glycosides (digoxin; average dose: 0.19±0.01 μg); and indirect anticoagulants (warfarin; average dose: 4.8±0.4 mg). Each anticoagulant prescription was individualized; the patient received a dose based on their International Normalized Ratio (INR) (Table 1).
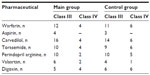  | Table 1 Routine treatment of CHF patients depending on NYHA class |
FSC therapy was carried out in combination with standard therapy for patients selected from the MG, after patients signed the informed consent, thus agreeing to treatment. A cryopreserved suspension containing pluripotent FSCs harvested from 5–9-week-old legally aborted embryonic cadavers was used for FSC treatment (FSCT). Aborted tissues were collected pursuant to ethical, moral, and legal principles of work with biological tissue. All donors were healthy women with negative test results for hemic infections.
One suspension was made of hematopoietic stem cells from fetal liver, while the other contained fetal brain stem cells and fetal heart stem cells.
The biotechnological process of suspension preparation included cell harvesting from germ layers, viability testing, programmed cryopreservation, and bacterial and viral safety testing.
Fetal material was harvested in the surgery room pursuant to all aseptic and antiseptic requirements. Tissue was collected (upon receiving written informed consent from the woman donor) from the liver, brain (nervous), and heart tissues of 5–9-week-old human fetuses aborted for family planning purposes, and they were found to have no developmental abnormalities or infections. Fetal tissue was then placed into sterile transport medium made of Hank’s Balanced Salt Solution and an antibiotic. Tissues were aseptically separated and homogenized in Hank’s solution. Water bath thawing of the cryopreserved suspension and viability testing were also performed right before FSCT.
Cryopreservation was performed with 5% dimethyl sulfoxide in three stages, with an initial temperature of 1°C/minute and crystal forming initiation. Preadministration cell viability was tested by trypan blue staining. Cells were counted in parallel in a Goryaev chamber (MiniMed Co, Ltd, Bryansk Town, Russian Federation) and in a TC10™ Automated Cell Counter (145-0001; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The stem cell viability before cryopreservation was 83.0%±3.0%. Right after cryopreservation at a low temperature (−196°C), and following water bath thawing of the cryopreserved suspension at 37.5°C±0.12°C, their viability was not less than 74.8%±1.03%.
In order to ensure safety, both the women donors and the ready-made hematopoietic stem cells from the fetal liver suspension were tested for bacterial and fungal safety, parasites, and viral infections such as human immunodeficiency virus 1 and 2, hepatitis B virus, hepatitis C virus, syphilis (Treponema pallidum), toxoplasmosis (Toxoplasma gondii), rubella, cytomegalovirus, herpes simplex virus 1 and 2, Epstein–Barr virus, Mycoplasma genitalium bacterium, Ureaplasma urealyticum, U. parvum, and Chlamydia trachomatis.
Suspensions containing hematopoietic stem cells from fetal liver, brain (nervous), and heart stem cells were stored in liquid nitrogen at −196°C in a properly arranged cryobank.
The stem cell procedure consisted of transplantation of the suspension containing cryopreserved FSCs preceded by premedication (infusion of 10 mg of diphenylhydramine and 15 mg of prednisone on day 1, and a specially prepared solution on day 2).
On day 1, we used hematopoietic stem cells from fetal liver harvested from the tissues of 5–9-week-old human fetuses. A suspension containing cryopreserved hematopoietic stem cells was administered via intravenous drip feed in the amount of 1.05±0.71 mL, with a nucleated cell count of 52.05×106/mL per transplantation. We also used homogenate-containing heart cell precursors, that were subcutaneously administered at 1.12±0.14 mL, with a nucleated cell count of 2.73×106/mL per transplantation.
On day 2, we subcutaneously administered ectodermal homogenate-containing nervous cell precursors in the amount of 2.12±0.49 mL, with a nucleated cell count of 7.9×106/mL per transplantation.
CD34+ were counted by flow cytofluorometry (Becton Dickinson, Franklin Lakes, NJ, USA) with fluorescent tagged antibodies (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Culture was made in 24well plates with 5% CO2 atmosphere in the air and 100% humidity at 37°C in the following semisolid medium: agar, 33% (Difco™; BD); glutamine, 4.0 mM/L (Sigma-Aldrich Co, St Louis, MO, USA); penicillin–streptomycin (100 U + 100 μg/mL) (Sigma-Aldrich Co); granulocyte–macrophage colony-stimulating factor, 100 U/mL (Sigma-Aldrich Co); interleukin-3, 100 U/mL (Behringwerke AG, Marburg, Germany); stem cell factor, 50 ng/mL (R&D Systems, Inc., Minneapolis, MN, USA); and erythropoietin, 10 U/mL (epoetin beta; Boehringer Ingelheim, Ingelheim, Germany). The media were prepared with Dulbecco’s Modified Eagle’s Medium (PAA Laboratories GmbH, Cölbe, Germany). The colony-forming unit (CFU) erythrocyte; CFU granulocyte monocyte; and CFU granulocyte, erythrocyte, monocyte, and megakaryocyte number and type of calculation was performed on the 14th day of culture growth.
Progenitor cells and stem cells of fetal neural and heart tissue were characterized by culturing them in vitro. This is supported by the fact that stem cells are received from the fetal brain and these cells have a tendency to differentiate exactly into the neuronal cells – axons and neurons. The heart obtained from the aborted material was separated from the other tissues. The caked blood was washed off. The isolated tissue was washed in Hank’s medium and antibiotics were aseptically homogenized in Hank’s solution. Following the routine procedure, the tissue was placed into fine ampoules which were exposed to cryopreservation.
CG patients underwent routine therapy alone, including muscle-supporting medications (L-carnitine, vitamins, lipoic acid, amino acids, vasoactive medications, and biostimulators), and medications that reduced muscle cell membrane damage (antioxidants).
All patients were re-examined 1 month, 3 months, and 6 months after treatment. The Duke Activity Status Index (DASI) scale was used to evaluate the patients’ physical capacity; their cognitive performance and emotional status were tested accordingly. Patients underwent 12-lead electrocardiography, echo Doppler, a 6-minute walking distance (6MWD) test, and NT-proBNP control.
A Schiller Cardiovit CS-100 cardiopulmonary cart instrument (Schiller AG, Baar, Switzerland) was used for electrocardiography and to record rhythm and conduction irregularities, LV hypertrophy, ST segment ischemia, Q-wave pathology, and nonspecific irregularities of the ST segment and T-wave.
Doppler echocardiography at rest was performed using an SSA-380A PowerVision 7000 recorder (Toshiba, Tokyo, Japan) at a variable frequency (2.5–3.5 MHz) in supine position, as well as on the left side, in accordance with the routine procedure.17 Systolic and diastolic functions of the heart ventricles and their remodeling were also examined.
The 6MWD test, expressed in meters, was performed and compared with the baseline 6MWD (i). Calculation of 6MWD (i) was made by the following formulas with respect to the patient’s age (years) and body mass index (BMI):
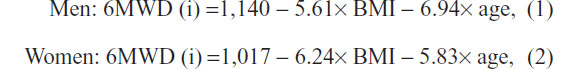
where BMI is the body weight (kg)/height2 (m) ratio.
The presence of NT-proBNP in the serum is indicative of early stages of cardiac failure and the most demonstrative marker of ventricular dysfunction.22 Its level correlates with CHF stage. An enzyme immunoassay was used for NT-proBNP testing.22
CF was measured by the MMSE scale, enabling the parallel evaluation of long- and short-term memory, speech, cognition, and constructional ability. Cognitive performance was assessed with the help of the Frontal Assessment Battery (FAB), including tasks for the evaluation of conceptualization, conflicting instructions, motor series (programming), and prehension behavior.23
Emotional instability and anxiety were detected through the State–Trait Anxiety Inventory, which is considered to be the most effective test for allowing the evaluation of both reactive (situational) anxiety (RA) at a certain moment and personal anxiety (PA) as a character trait.23 Depression was assessed by means of Beck’s Depression Inventory.
The significant difference between mean values was calculated by Student’s t-test (for parametric statistics) and the Mann–Whitney test (for independent samples). The difference was regarded as statistically significant if P<0.05. The data were processed using Statistica version 8.0 software (StatSoft, Inc., Tulsa, OK, USA). This study was approved by the local ethical committee.
Results
Within 1 month after FSCT, all MG patients with NYHA class IV (four patients, or 20%) were downgraded to NYHA class III, and five patients with NYHA class III (25% from the total number, or one- quarter of patients with NYHA class III) were downgraded to NYHA class II. Thus, 1 month after FSCT, none of the patients were classified as NYHA class IV CHF, while 15 and five patients manifested NYHA class III (75%) and NYHA class II (25%) CHF, respectively. Three months after FSCT, the number of patients with NYHA class III CHF was reduced by four (to eleven patients), and nine patients showed NYHA class II CHF. Six months after FSCT, eight and 12 patients scored as NYHA class III (40%) and NYHA class II CHF (60%), respectively.
As for the CG, within 1 month after FSCT, three patients with NYHA class IV (15% from the total number of class IV patients) were downgraded to NYHA class III, and four patients with NYHA class III (20%) were downgraded to NYHA class II. Three months after FSCT, three remaining patients with NYHA class IV CHF were downgraded to NYHA class III; the number of NYHA class III patients was reduced by three (to 13 patients, or 65%). Seven (35%) patients showed NYHA class II CHF and none had NYHA class IV CHF. Six months after FSCT, eleven (55%) and nine (45%) patients scored as NYHA class III and class II CHF, respectively. The difference in the number of patients with NYHA class II, class III, and class IV at 1 month, 3 months, and 6 months after FSCT was insignificant both in the MG and the CG (P>0.05); however, the tendency for the NYHA class to downgrade, as well as its rate, can be clearly traced (Table 2).
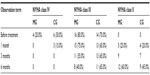  | Table 2 NYHA dynamics after routine treatment with/without FSCs |
The next phase of the study followed the life quality changes in CHF patients on the DASI scale, physical tolerance (6MWD), and the morphologic–functional parameters of the LV myocardium (Table 3).
The treatment resulted in improvements of the studied parameters – increases in the DASI score, physical tolerance (6MWD), and LVEF, as opposed to EDV reduction.
Within 1 month after FSCT, the MG DASI score increased by 54.6%; after 3 months, it increased by 63.2%. After 6 months, it increased by 66.4%, which is significantly higher than baseline (P<0.05 versus baseline). At the same time, the CG DASI score increased by 10.7% 1 month after treatment; in 3 months it increased by 38.1% (P<0.05), and in 6 months it increased by 41.7%, which is significantly higher in comparison with baseline (P<0.05). A comparative analysis of the results in both groups revealed that within 1 month after treatment, the significant increase of the DASI score in the MG was fivefold higher than in the CG, and 6 months after treatment, the significant difference between groups was preserved (P<0.05) – it was 30% higher in the MG. Thus, in both groups, the functional capacity index significantly improved over the 6-month period after treatment, but the improvement rate was quicker (1 month after treatment) and more intensive in the group that underwent FSCT.
In the MG, significant changes on the 6MWD were reported as early as 1 month after the combined treatment with FSCs; the distance walked increased 7.3-fold (P<0.001), 10.3-fold (P<0.001), and 12.5-fold (P<0.001) at 1 month, 3 months, and 6 months, respectively. In the CG, the test results also improved as the distance walked increased 5.6-fold (P<0.001), 7.1-fold (P<0.001), and 9.5-fold (P<0.001) at 1 month, 3 months, and 6 months, respectively. A significant difference between the groups was obvious as early as 1 month after the treatment; in the MG, the result was higher by 24.1% (P<0.001), 34.2% (P<0.001), and 23.9% (P<0.001) at 1 month, 3 months, and 6 months, respectively.
In the MG, the LVEF remained almost unchanged during the first month after FSCT. However, it increased by 10.5% (P<0.05) and by 20.9% (P<0.05) over 3 months and 6 months after FSCT, respectively, in comparison with baseline, and by 9.9% (P<0.5) in comparison with the 3-month result. In the CG and MG patient, the LVEF remained practically unchanged within 1 month and 3 months versus baseline. After 6 months, it increased by 10.1% in comparison with baseline (P<0.05), but it was 9.6% lower in comparison with the MG (P<0.05).
In the MG, the LV EDV decreased as early as 1 month after FSCT, and it continued to drop afterwards – by 7.85% (P<0.05), 13.51% (P<0.01), and 20.51% (P<0.01) at 1 month, 3 months, and 6 months, respectively. In the CG, the LV EDV decreased by 9.05%, 12.5%, and 17.7% (P<0.05) at 1 month, 3 months, and 6 months after treatment, respectively. Moreover, a significant difference (P<0.05) between the groups was obvious 6 months after treatment, in an average of 23.3%. In the MG, the LV EDV reduction was more intensive than in the CG, though it was insignificant.
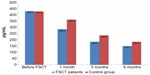
In the MG, serum NT-proBNP significantly decreased within 1 month after FSCT (by 33.75%, 57.2%, and 65.13% at 1 month, 3 months, and 6 months, respectively; P<0.001 for all) (Figure 1). Routine treatment also resulted in an NT-proBNP reduction of 15.3%, 44.9%, and 64.9% at 1 month, 3 months, and 6 months, respectively, versus baseline (P<0.001 for all) (Figure 1).
It is obvious that serum NT-proBNP in the MG decreased at a quicker rate than in the CG – by 21.2%, 27.8%, and 18.5% at 1 month, 3 months, and 6 months, respectively (P<0.05 for all). Thus, with routine therapy, the NT-proBNP decreased in both groups; however, in the MG, it was significantly higher starting from month 1 and this continued throughout the entire observation period.
Clinical improvements, both objective and subjective, were reported in all CHF patients. A significant increase in the LVEF by 20.9% (P<0.05) and an LV EDV reduction by 20.51% (P<0.05) were reported 6 months after FSCT. NT-proBNP was reduced by 33.75%, 50.0%, and 65.13% (P<0.001 for all) over 1 month, 3 months, and 6 months after FSCT. The 6MWD test results increased 7.3-fold, 10.3-fold, and 12.5-fold over 1 month, 3 months, and 6 months after FSCT, respectively (P<0.01 for all). Within 1 month after the treatment, the DASI score increased twofold, and after 6 months, it increased by 66.4% (P<0.01 for both).
A comparative analysis of the MG and the CG revealed changes in the functional capacity and in the morphofunctional parameters of the LV myocardium. As early as 1 month after treatment, the DASI score in the MG was fivefold higher than in the CG. The 6MWD score and NT-proBNP improved in both groups within 1 month after treatment, but in the MG, these improvements were more intensive. In the MG, the LVEF increased (versus baseline) within 3 months, while in the CG, this was reported only at 6 months, and the extent of improvement was the same as in the MG after 3 months. The LV EDV reduced in all patients within 1 month after treatment, but the difference between the groups became significant after 6 months.
The CF in both groups was assessed on the MMSE and FAB. The pretreatment CF analysis revealed that the MMSE and FAB scores were 24.2±0.31 and 14.1±0.21, respectively, for NYHA class II MG patients, and 24.9±0.19 and 14.9±0.42, respectively, for the CG. For the MG class III patients, the scores were 23.6±0.22 and 13.5±0.16, respectively, and 24.1±0.12 and 14.2±0.23, respectively. For the MG class IV patients, the MMSE and FAB scores were 22.8±0.12 and 12.9±0.34, respectively, as well as 21.5±0.24 and 13.6±0.44, respectively, for the CG. In other words, the higher the NYHA class, the lower the observed CF.
The average MMSE score at baseline in the MG was 23.89±0.51, and 23.43±0.59 in the CG. One month after FSCT, no difference was seen on the MMSE versus baseline in either group. A significant increase in the general score (to 25.7±0.24), in the attention and calculation score (to 4.1±0.23), and in the recall score (to 2.1±0.34) (P<0.05 for all) was reported in the MG 3 months after treatment. Similarly, 6 months after FSCT, a significant increase of the general score and across the MMSE subtests were also reported in the MG. The general score increase was reported in the CG only 6 months after treatment (24.79±0.55) but, in comparison with baseline, this difference was insignificant (P>0.05) (Figure 2).
The average FAB score at baseline in the MG was 13.32±0.47, whereas in the CG it was 13.29±0.40 (P>0.05). In the MG, the degree of frontal dysfunction (in terms of conceptualization, programming, inhibitory control, and conflicting instructions) on the FAB scale remained unchanged 1 month after FSCT while, at the same time, lexical fluency improved somewhat (from 2.3±0.58 to 2.7±0.47; P>0.05), though the general score was practically unchanged. Three months after FSCT, the conceptualization score increased to 2.7±0.23 (P<0.05), and the programming score increased to 3.0±0.12 (P<0.05). A significant (P<0.05) increase in the average score and across all areas on the FAB was also reported 6 months after FSCT (Figures 3 and 4). No significant mean FAB score increase was reported after 6 months in the CG.
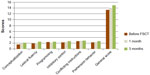  | Figure 3 Cognitive performance on the FAB scale in patients before FSCT, and over 1 month and 3 months after FSCT. |
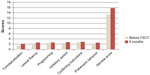  | Figure 4 Cognitive performance on the FAB scale in patients before treatment and over 6 months after FSCT. |
Thus, a significant improvement of the general MMSE score and all cognitive subtest scores (recall, attention and calculation, orientation, and praxis) starting from the third month after treatment, as well as an improvement in the FAB score (conceptualization, lexical fluency, inhibitory control, conflicting instructions, and prehension behavior) was reported in the MG 6 months after FSCT. No significant increase (versus baseline) was reported in the CG 6 months after treatment. Emotional changes and depression development in dynamics among the patients of both groups are shown in Table 4.
Thus, the FSCT resulted in a significant subsidence of depression and RA, as the CHF patient’s reaction to the disease (P<0.05) in the MG within 1 month after treatment versus baseline was maintained over 3 months and 6 months (P<0.05). The PA level also decreased, but its reduction became significant only 6 months after the treatment. The situation in the CG was different; the RA significantly reduced within 6 months after the beginning of treatment versus baseline (P<0.05). However, at the same time, the PA reduction was significant over 3 months and 6 months after the treatment versus baseline (P<0.05). In the MG, depression significantly subsided as early as over 1 month after treatment, while in the CG, significant subsidence started from the third month after treatment.
Although a 6-month follow-up period is not sufficient for making recommendations regarding wide clinical applications, it is notable that no side effects affecting the cardiovascular system or brain functions have been reported during the entire observation period among CHF patients treated routinely in combination with FSCT. Moreover, there were no cases of treatment-induced allergies, which is indicative of an absence of contraindications for FSCT. Thus, the present FSCT method can be regarded as a safe and promising protocol for further long-term follow up, and it is recommended for subsequent clinical application.
Discussion
In CHF, FSCT was used to improve the heart’s contractile activity and LV remodeling process regulation through the substitution of cells that were unable to contract, or by substituting those tissues that were necrotic or sclerotic. In our opinion, the functional restoration of the myocardium can be reached through an increase in the number of cells capable of contraction in the myocardium, and/or through increasing the functional reserve of the patient’s cardiomyocytes via stimulation of intracellular regeneration processes.11,16,24 It is common knowledge that cardiomyocytes are electromechanically integrated through special contacts in the intercalated discs of cardiac muscle containing fascia adherens (a myofibril adherence area), gap junctions, and desmosomes.4,10,25 Desmosomes and fascia adherens perform mechanical functions or, in other words, they fix cardiomyocytes, while gap junctions conduct electric impulses. Electromechanical integration of cardiomyocytes is of paramount importance for coordinated activity of the myocardium, making cardiac muscle work as an integral unit. Taking into consideration all of these factors, the success of cardiomyocyte transplantation is defined by whether it will be capable of interacting with the muscle cells of the recipient and with the microenvironment.14
FSCs are more specialized than embryonic stem cells; moreover, they show higher proliferative and expansion potential and lower immunogenicity than other stem cell types.26 FSCs are more rapidly, easily, and efficiently reprogrammed to pluripotency than are neonatal and adult cells.27 In addition, they exert strong immunomodulation effects, possess a stable phenotype, and demonstrate less senescence.28 In addition, FSCs – unlike embryonic stem cells – have no potential to form in vivo tumors, still retaining their primitive properties and expansion capacity.27 At 4–5 days of embryonic development, at the blastocyst stage of its inner cell mass, embryonic stem cells can be isolated.29 Subsequently, after this division, FSCs can be isolated. Indeed, by days 7–10, gastrulation occurs and the embryonic cells have committed to a specific lineage: meso; ecto; or endo. By 4 weeks, the neural tube has formed, and by 5–7 weeks, the major organs are all formed.29 We used FSCs from 5–8 weeks. The FSC transplantation we performed was safe and well tolerated. No immediate or long-term side effects, or immunological reactions, were observed during the 1-year follow-up period.
The experimental use of human fetal tissues is a very sensitive and controversial issue. It is not supported, and it is even prohibited in certain countries, including in the United States and the European Union.8 In other countries, including the Ukraine, this biological material can be used for experimental purposes upon the consent of the woman making the decision about the abortion and donation of the fetal material in accordance with ethical, moral, and legal principles. There are many opponents of the use of the aborted human embryonic tissues for medical purposes because, in their opinion, this will result in a growing “demand” for abortions.8 In our emancipated times, when most women are working and holding top positions, they wish to plan their lives and decide about the timing of childbirth. Therefore, in spite of the protests of some political and religious organizations, abortions will not stop. Taking into consideration the value of fetal material with its huge potential of helping many people, even those with incurable diseases, it would be inhumane not to use this opportunity to save or improve people’s lives. In view of the small, but very promising experience of the use of FSCs that are capable of differentiating into different functional specialized cells, scientists lay great hopes for the effective clinical use of these cells.8,30,31
Apart from the aforementioned issues, some experts fear undesirable adverse effects of FSCT. According to the literature,26,32 wrong differentiation of embryonic stem cells can result in tumor (teratoma) formation. In our study, we used FSCs that, unlike embryonic cells, are pluripotent and not totipotent, and as a consequence, they exert no cancerogenic properties. Another discussed adverse effect of FSCT is rejection; however, due to the immaturity of human leukocyte antigen receptors in 5–9-week-old fetuses,27,33,34 rejection does not develop and was not reported by any patient during the observation. Our preliminary conclusion is, therefore, that routine therapy in combination with FSCs is a promising and safe method for CHF treatment.
The results bring hope for the combined treatment of CHF with routine methods and FSCT, and they possibly allow for FSCT as a future alternative to heart transplantation. Obviously, this concerns, first of all, the patients on waiting lists for heart transplantation. It is too early to make any assumptions regarding the potential role of FSCT in the prevention of cardiomyocyte apoptosis, but there is hope that studies in progress will shed light on this aspect of the effect of FSCT on the course and prognosis of CHF.
Conclusion
Combined CHF treatment with routine methods and FSCs resulted in improved contractile activity of the myocardium (the LVEF increased by 20.9% within 6 months versus baseline, which is twofold higher than in CHF patients who were treated without FSCT) and LV remodeling (LV EDV decreased by 20.51% within 6 months versus baseline). The biological CHF marker – NT-proBNP – dropped by 33.75% within 1 month after FSCT, by 57.2% within 3 months, and by 65.1% over 6 months (P<0.001 for all).
CHF patients reported significant functional and life quality improvements. The 6MWD test results, in comparison with baseline, increased 7.3-fold, 10.3-fold, and 12.5-fold over 1 month, 3 months, and 6 months, respectively, which is twofold higher than in the CG. The DASI score increased by 54.6%, 63.2%, and 66.4% over 1 month, 3 months, and 6 months after treatment, respectively. A comparative analysis between groups revealed significant increases of the DASI score in the MG as early as within 1 month (P<0.05), which is fivefold higher than in the CG within the same period of time.
Cognitive performance improvement was observed on the MMSE (recall, attention and calculation, orientation, and language) and across all areas of the FAB (conceptualization, lexical fluency, inhibitory control, conflicting instructions, and prehension behavior) starting from the sixth post-treatment month.
RA reduction started 3 months after the FSCT, while PA was significantly reduced within 6 months after treatment. Depression significantly subsided as early as over 1 month after treatment.
It has been proven that FSCT is a safe and effective therapeutic method that can be used in advanced CHF. This method offers great hope but, at the same time, it calls for controlled randomized clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
Zakharov VV, Yakhno NN. [Moderate cognitive impairment in the elderly: diagnostics and treatment]. Russian Medical Journal. 2004;10:573–576. Russian. | |
Levin OS. Dyscirculatory encephalopathy: modern knowledge about development mechanisms and treatment. Consilium Medicum. 2007;8:72–79. | |
Enright PL, Sherill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. | |
Robbins MA, O’Connell JB. Economic impact of heart failure in management of end-stage heart disease. In: Rose EA, Stevenson LW, editors. Management of End-Stage Heart Disease. Philadelphia, PA: Lippincott-Raven; 1998:3–11. | |
Wang JS, Shum-Tim D, Chedrawy E, Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122(4):699–705. | |
Ostroumov EN, Yermolenko AE, Gureev SV, et al. [Right ventricle ejection fraction as myocardium revascularisation efficiency marker in ischemic heart disease with congestive circulatory failure]. Cardiology. 1996;4:57–61. Russian. | |
Pullicino PM, Hart J. Cognitive impairment in congestive heart failure?: Embolism vs hypoperfusion. Neurology. 2001;57(11):1945–1946. | |
Petrenko AY, Khunov YA, Ivanov YN. Stem Cells. Properties and Clinical Perspectives. Luhansk, Ukraine: Press Express; 2001:224–239. | |
Acanfora D, Trojano L, Iannuzzi GL, et al. The brain in congestive heart failure. Arch Gerontol Geriatr. 1996;23(3):247–256. | |
Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(Suppl 19):II247–II256. | |
Lainchbury JG, Troughton RW, Frampton CM, et al. NTproBNP-guided drug treatment for chronic heart failure: design and methods in the “BATTLESCARRED” trial. Eur J Heart Fail. 2006;8(5):532–538. | |
Trojano L, Antonelli Incalzi R, Acanfora D, et al; Congestive Heart Failure Italian Study Investigators. Cognitive impairment: a key feature of congestive heart failure in the elderly. J Neurol. 2003;250(1):1456–1463. | |
Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–1918. | |
Tomita S, Mickle DA, Weisel RD, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123(6):1132–1140. | |
Jefferson AL, Benjamin EJ. Cardiovascular disease, cognitive decline, and dementia. In: Wahlund LO, Erkinjuntti T, Gauthier S, editors. Vascular Cognitive Impairment in Clinical Practice. Cambridge, UK: Cambridge University Press; 2009:166–177. | |
Ohno N, Fedak PW, Weisel RD, Komeda M, Mickle DA, Li RK. Cell transplantation in non-ischemic dilated cardiomyopathy. A novel biological approach for ventricular restoration. Jpn J Thorac Cardiovasc Surg. 2002;50(11):457–460. | |
Feigenbaum H, editor. Echocardiography. 5th ed. Philadelphia, PA: Lea and Febiger; 1994. | |
Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. | |
Taupin P. Stem Cells and Regenerative Medicine: Volume III: Pharmacology and Therapy. New York, NY: Nova Science Publishers; 2008. | |
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. | |
Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. | |
Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: Feasibility and potential clinical advantages. J Thorac Cardiovasc Surg. 2000;120(5):999–1006. | |
Vogels RL, Oosterman JM, van Harten B, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24(6):418–423. | |
Cacciatore F, Abete P, Ferrara N, et al. Congestive heart failure and cognitive impairment in an older population. Osservatorio Geriatrico Campano Study Group. J Am Geriatr Soc. 1998;46(11):1343–1348. | |
Sakakibara Y, Tambara K, Lu F, et al. Combined procedure of surgical repair and cell transplantation for left ventricular aneurysm: an experimental study. Circulation. 2002;106(12 Suppl 1):I193–I197. | |
Shumakov VI, Onischenko NA, Krasheninnikov ME, et al. The stromal bone marrow stem cells differentiation into cardiomyocyte cells in various mammalian species. Bulletin of Experimental Biology and Medicine. 2003;4:461–465. | |
Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321–334. | |
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. | |
Panteghini M. Recommendations on use of biochemical markers in acute coronary syndrome: IFCC proposals. eJIFCC, no 14, 2004. Available from: http://www.ifcc.org/ifcc-communications-publications-division-%28cpd%29/ifcc-publications/ejifcc-%28journal%29/e-journal-volumes/vol-14-n%C2%B0-2/recommendations-on-use-of-biochemical-markers-in-acute-coronary-syndrome-ifcc-proposals/. Accessed July 17, 2014. | |
Anderson A, Nielsen JM, Peters CD, Schou UK, Sloth E, Nielsen-Kudsk JE. Effects of phosphodiesterase-5 inhibition by sildenafil in the pressure overloaded right heart. Eur J Heart Fail. 2008;10(2):1158–1165. | |
Georgiadis D, Sievert M, Cencetti S, et al. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J. 2000;21(5):407–413. | |
Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–280. | |
Nichols WW, O’Rourke MF. Aging, high blood pressure and disease in humans. In: Nichols WW, O’Rourke MF, editors. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 3rd ed. London, UK: Edward Arnold; 1990:398–420. | |
Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.