Back to Journals » Psoriasis: Targets and Therapy » Volume 4
Epidemiology and treatment of psoriasis: a Chinese perspective
Received 4 May 2014
Accepted for publication 17 June 2014
Published 13 October 2014 Volume 2014:4 Pages 37—47
DOI https://doi.org/10.2147/PTT.S51717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Ran Pan, Jianzhong Zhang
Department of Dermatology, Peking University People's Hospital, Beijing, People's Republic of China
Background: Psoriasis is a chronic inflammatory skin disease that has a negative impact on quality of life. Prevalence and management of psoriasis varies among different ethnic groups.
Objectives: To evaluate the epidemiology and treatment of psoriasis from a Chinese perspective.
Methods: A systematic search was performed on PubMed and the China National Knowledge Infrastructure using the following MeSH terms: "psoriasis" and ("prevalence" or "epidemiology") and "risk factor" and ("management" or "treatment"). The search included all citations from 1975 to 2013. Data were sorted by prevalence, age of onset, sex distribution, type, severity, risk factors, and management and treatment. Severity of psoriasis was classified as mild, moderate, or severe. The studies cited in this review involved Chinese subjects.
Results: The prevalence of psoriasis in the People's Republic of China ranged from 0.11% to 0.47%. Genetic and environmental factors played an important role in initiation and exacerbation of psoriasis. Results showed that psoriasis can occur at any age but is more common in young and middle-aged individuals and occurs more often in men and earlier in women. Psoriasis vulgaris accounted for 82.6%–97.1% of psoriasis patients. More than 90% of patients with psoriasis were classified as mild or moderately severe. Risk factors are numerous. Management and treatment was based on classification level.
Conclusion: The prevalence of psoriasis in Chinese patients is lower than that in Caucasians. A cold and dry climate, bacterial infection, diet, and stress are important risk factors for developing psoriasis. There are a variety of management and treatment options available. As such, Chinese patients with psoriasis can receive effective, safe, and individualized treatment.
Keywords: psoriasis, epidemiology, risk factors, treatment
Introduction
Psoriasis is a chronic inflammatory disease. Although most patients present with skin and nail manifestations, some develop extracutaneous manifestations such as psoriatic arthritis. Patients with psoriasis have a high risk of developing cardiovascular disease, diabetes, metabolic syndrome, and depression.1–4 Although the etiology and pathogenesis of psoriasis remain unclear, it has been suggested that hereditary and environmental factors play pivotal roles in psoriasis. Psoriasis has a significant impact on quality of life, even in patients whose affected body surface area is relatively small.5 It is essential that each psoriasis patient receives effective, individualized, and safe treatments. This review focuses on the prevalence and treatment of psoriasis from a Chinese perspective.
Methods
Literature on psoriasis prevalence and management from 1975 to 2013 were obtained from PubMed and the China National Knowledge Infrastructure. The following MeSH terms were used: “psoriasis” and (“prevalence” or “epidemiology”) and “risk factor” and (“management” or “treatment”). All the Chinese clinical trials cited in the manuscript were performed on Chinese patients.
Prevalence
Prevalence of psoriasis varies among ethnic groups worldwide. Prevalence in Europe, North America, and Australia is higher than in Asia, and is lowest in South America and West Africa. In Europe, the prevalence of psoriasis has been shown to range between 2.0% and 6.5%.6 The highest prevalence (11.8%) was reported in the city of Kazach’ye in the Arctic region of Russia.7 In North America, the prevalence ranges between 2.2% and 4.7%. Many psoriasis surveys have been conducted in Asia. The reported prevalence of psoriasis is 0.5%–1.5% in India, 4%–5.5% in Malaysia, 0.29%–1.18% in Japan, and 3.1% in Kuwait.7 Several Chinese studies have examined the prevalence of psoriasis in different regions of the People’s Republic of China (Table 1). In 1978, a study was performed in Xinjiang Province where the prevalence of psoriasis was 0.18%.8 A large-scale questionnaire-based survey was conducted in the People’s Republic of China in 1984: results showed a prevalence of 0.12%.9 In 2001, another survey was conducted in the rural areas of Anhui province and revealed a prevalence of 0.11%.10 In 2012, a large-scale population-based survey was conducted in six Chinese provinces, showing a prevalence of 0.47%.11 Compared to these questionnaire-based surveys, the current study was questionnaire-based followed by dermatologist confirmation of the veracity of the study. In 2013, a study in Hainan province reported a prevalence of 0.14%.12
  | Table 1 Prevalence of psoriasis in the People’s Republic of China |
Age of onset
Although psoriasis can occur at any age, it is more common in young and middle-aged individuals and occurs earlier in women than in men. Henseler and Christophers13 classified psoriasis into two types based on age of onset. Patients who developed psoriasis before age 40 years were defined as Type I psoriasis. These patients had higher incidences of the heavy chain receptors HLA-Cw6, -B57, and -DR7. Patients with Type II psoriasis developed the disease after age 40 years and their disease was not associated with HLA.13 Type I psoriasis is usually more severe than Type II. In the 1984 survey, ~75% of Chinese patients developed psoriasis before age 34 years.9 Peak onset age in women was 5 years earlier than for men. In the 2012 study, there were two peak onset ages. One was at 20–29 years and another at 40–49 years. Approximately 68% of patients developed psoriasis before age 40 years and onset was similar for men and women.11
Sex distribution
Discrepancies regarding the prevalence of psoriasis in men and women have been reported. Some studies showed a similar prevalence between the two sexes.14,15 A Chinese study by Xu et al reported that prevalence of psoriasis was 0.12% in men and 0.11% in women.10 Other studies showed a higher prevalence in men. An Indian study reported that the prevalence of psoriasis in men was twice that of women.16 Three Chinese studies showed that the prevalence of psoriasis was higher in men. The 1984 study indicated a prevalence of 0.19% versus (vs) 0.14% for men and women, respectively.9 The 2012 study indicated a prevalence of 0.54% vs 0.44% for men and women, respectively,11 and the 2013 study revealed that prevalence of psoriasis in men was twice that of women (0.19% vs 0.08%).12 Other studies showing a male predominance for psoriasis have been reported in Chinese and Japanese studies.17–19
Types
Chinese studies show that psoriasis vulgaris accounts for 82.6%–97.9% of psoriasis patients (Table 2). Pustular psoriasis, psoriasis arthropathica, and erythrodermic psoriasis account for 0.69%–2.17%, 0.69%–6.52%, and 0%–8.7%, respectively.9,11,12 The incidence of psoriasis vulgaris and pustular psoriasis in the People’s Republic of China is similar to that reported in Japan (Table 3), while psoriasis arthropathica and erythrodermic psoriasis are slightly higher.18,19 Compared to the high incidence of psoriasis arthropathica in Caucasians, accounting for 30% of psoriasis incidence,20 the incidence of psoriasis arthropathica in Chinese patients is lower. It is also lower in Middle-Eastern and other Asian countries. It accounts for 9% in Korea, 9% in Iran, 2% in Turkey, and 1% in Japan.18,21–23
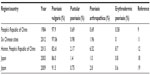  | Table 2 Different types of psoriasis identified in Chinese and Japanese studies |
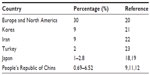  | Table 3 Percentage of psoriasis arthropathica in different studies |
Severity
Severity of psoriasis is usually assessed by body surface area (BSA). Lesions affecting <3% of BSA are classified as mild, 3%–10% as moderate, and >10% as severe psoriasis. In the 1984 survey, 63% of subjects had mild psoriasis, 29% had moderate psoriasis, and 7.71% had severe psoriasis.9 In the study performed in Liaoning province, 22.2% of cases were mild, 70.2% moderate, and 7.5% severe.17 These results indicate that >90% of subjects with psoriasis were classified as mild or moderately severe.
Risk factors
Genetics
Genetic factors play an important role in psoriasis. A study involving 8,045 twins in Norway reported that the prevalence of psoriasis in identical twins was much higher than in fraternal twins.24 Kundakci et al reported a higher incidence of psoriasis in male subjects based on inheritance.23 Jesic et al reported that mother–son inheritance was more common than mother–daughter and father–children inheritance.25 In the People’s Republic of China, 22%–32% of subjects with psoriasis had a positive family history and had earlier onset than sporadic psoriasis.9–11,17 The 1984 study showed that heritability of psoriasis was 4.6% in first degree relatives, which was 22 times that of the common population (0.21%).9 In the 2012 study, 28% of subjects had a positive family history of ~13 times that of the common population.11 Zhang et al reported that 29.4% of subjects with psoriasis vulgaris had a positive family history. Also, patients born to psoriatic parents had earlier onset than those born to healthy parents. The heritability of psoriasis was 71% in first degree relatives and 37% in second degree relatives.26
Drugs
Drug-induced psoriasis is characterized by occurrence of psoriasis in patients with no previous incidence or family history of psoriasis following administration of medications, and cessation of disease after discontinuation of medication. Jiang reported that medication was a risk factor for psoriasis.27 Tan Pei Lin et al reported that two Chinese women developed psoriasis after taking fluoxetine for 10–18 months.28 A similar case was reported after use of ampicillin.29 Medications that have been reported to induce psoriasis include chlorpromazine, procaine, rabies vaccine, and hepatitis B vaccine.30–33
Medications can also exacerbate existing psoriasis.34 Patients with medication aggravating psoriasis have usually been previously diagnosed with, or have a family history of, psoriasis. In these patients, medications can induce new lesions or exacerbate pre-existing ones. These drugs include β-blockers, lithium, antimalarial, non-steroidal anti-inflammatory drugs, and angiotensin-converting enzyme inhibitors. Others include calcium channel blockers, interferon, terbinafine, diazepam, and antibiotics (especially tetracycline).35–38
Weight
A prospective study by Setty et al39 suggested that women with a higher body mass index (BMI) were more prone to psoriasis; this finding was confirmed by a study by Bryld et al.40 Zhang et al studied 4,452 subjects with psoriasis vulgaris and 1,166 healthy individuals, and found that overweightness and obesity occurred in 23% and 4% of subjects with psoriasis, respectively, which was significantly higher than controls. Severity of psoriasis and Psoriasis Area and Severity Index (PASI) scores were correlated with BMI.41 Another Chinese study showed that 28.7% of subjects with psoriasis had a high BMI (BMI >25). Most subjects were adult women with late onset and more severe disease.42 Miller et al found that ethnicity did not influence the link between obesity and psoriasis.4 Obesity was reported to reduce the reaction to systemic therapy and the retention rate of tumor necrosis factor (TNF)-alpha blockers.43,44 Weight reduction can improve sensitivity to low-dose cyclosporine.45
Smoking
It has been reported that smoking and having previously smoked are risk factors for psoriasis.46 Li et al demonstrated that smoking is an independent risk factor for psoriasis in both men and women.47 Zhang et al showed that more male smokers developed psoriasis than non-smokers and frequency of smoking was associated with severity of psoriasis.48
Stress
Stress is known to be an important risk factor for developing psoriasis. In the current study, more than 60% of subjects complained of exacerbation of psoriasis following stressful events such as divorce, loss of job, loss of family member, and severe disease. A prospective study showed that severity of psoriasis was associated with severity of stress.49 Stress not only leads to clinical exacerbation but also has negative effects on efficacy of therapy. Fortune et al reported that stress can decrease the efficacy of psoralen ultraviolet A (PUVA).50
Climate
Studies have shown that psoriasis occurs more frequently in cold, dry climates compared to warm, humid climates. In the People’s Republic of China, the prevalence of psoriasis was reported higher in Northern (cold and dry) than in Southern (warm and humid) regions.9 It has been found that latitude and pollution are positively correlated with psoriasis, while air temperature and humidity are negatively correlated. Latitude had the most impact on prevalence of psoriasis, followed by air temperature and humidity.51 However, Jacobson et al reported that latitude was not an independent risk factor for psoriasis.52
Seasonal remission and exacerbation of psoriasis are common. It has been shown that exacerbations increase in the spring.9 A study in Liaoning province showed that most psoriasis subjects experienced exacerbations during winter and spring, although first onset of the disease occurred in any season.17 A study in Anhui province showed similar findings.10
Diet
Diet is another risk factor that has been shown to induce and aggravate psoriasis. A recent Korean study showed that diet is an important regulatory factor of immune response. The intake of polyunsaturated acids was inversely associated with biomarkers of inflammation (C-reactive protein [CRP], interleukin [IL]-6, and TNF-α), while intake of trans and saturated acids was positively associated with levels of CRP and IL-6.53 Wei et al studied the relationship between food and psoriasis in 646 psoriasis subjects and 647 healthy controls.54 Results showed that alcohol consumption, spicy foods, and fat-rich meat were risk factors for psoriasis (P<0.01), while protein-rich meat and vegetable oil were protective factors (P<0.01). Tong et al reported that seafood, alcohol, and pungent foods were risk factors for psoriasis.55 However, a study by Ding et al found no correlation between food and psoriasis exacerbations.11
Infection
Infections are an important trigger of guttate psoriasis, especially streptococcal infections.56 Tonsillectomy has been considered a treatment for guttate psoriasis, palmoplantar pustulosis, and plaque psoriasis.57 A Spanish study reported a group of young subjects (21–40 years) who had upper respiratory tract infections before onset of psoriasis.58 Some Chinese studies have shown similar findings.9,12,17 Infection is the most common trigger for childhood psoriasis, which is consistent with the findings of Wu et al and Chiam et al regarding Chinese, Dutch, and Singaporean psoriasis studies.59,60 Antimicrobial treatments are very important in these patients.
Management
In 2008, the Chinese Society of Dermatology created guidelines for the management of psoriasis.61 They include the following: treatment objectives should include prevention of disease progression, attenuation of symptoms, relapse avoidance, reduction in treatment side effects, and improvements in quality of life; clearance of rash is not essential; assessment of psoriasis and effective communication with the patient are important prior to treatment initiation; the advantages and disadvantages of each therapy should be considered and discussed with the patient; and finally, patients should receive individualized treatment according to type and severity of the disease.
Severity of psoriasis is usually measured by BSA. Mild disease is defined as a BSA score <3% and severe disease as a BSA score >10%. A moderate BSA score ranges from 3% to 10%. Mild psoriasis is usually treated with topical agents, sometimes combined with phototherapy. Moderate and severe psoriasis are treated with combined topical and systemic therapy. If the effects of topical agents and/or phototherapy are not satisfactory, addition of systemic agents should be considered. Biologics have recently been widely used to treat severe psoriasis. In the People’s Republic of China, biologics are usually used when patients fail to respond to conventional systemic treatment and patient quality of life is seriously affected.
For mild psoriasis, topical corticosteroids and vitamin D3 analogs are the mainstay of treatment. For moderate to severe psoriasis, ultraviolet B (UVB) therapy is widely used. Acitretin, cyclosporin A (CsA), and other immunosuppressive agents are used only in severe cases. Etanercept and infliximab have also been successfully used in patients with severe psoriasis. Adalimumab and ustekinumab have completed clinical trials and results will soon be available in the People’s Republic of China.
Topical treatments
Topical agents are fundamental in the treatment of psoriasis. They target the affected skin directly and have the advantage of being effective, safe, and well tolerated. Mild disease can be successfully treated by topical treatment alone, while moderate and severe disease can be controlled with combined phototherapy or/and systemic therapy. Since topical treatments often require long-term use, it is suggested that patients receive an individualized treatment that is adjusted over time as required. Type of topical agent should be considered. Creams and ointments are the most common formulations for epidermal psoriasis. Solutions and lotions are regularly used for scalp psoriasis. For very thick lesions, short-term plaster or occlusive treatment is often used. In the People’s Republic of China, most psoriasis patients are classified as mild to moderate severity. As such, topical treatment is most often used; topical corticosteroid is the most frequently prescribed medication for psoriasis patients. Topical vitamin D analogs, retinoids, and tacrolimus ointment are also frequently used.
Emollients
Use of an emollient is an essential part of routine psoriasis treatment.61 Emollients moisturize the skin, thereby promoting healing and improving skin barrier function. They also help control pruritus and reduce scaling. Addition of an emollient can help taper the dose and side effects of other topical agents. Hu studied the efficacy of topical tacalcitol in combination with an emollient and tacalcitol as monotherapy for psoriasis vulgaris. After 4 weeks of treatment, 85.2% of subjects in the combined therapy group and 64.7% in the monotherapy group achieved good responses.62 A study by Qi and Liu evaluated the use of twice daily emollient compared to twice daily retinoid ointment in the treatment of psoriasis vulgaris. After 2 months, efficacy rates were 86.7% and 70.0% in the emollient and retinoid ointment groups, respectively, indicating that emollient was more effective than retinoid ointment.63 Wang et al treated subjects with narrowband (NB)-UVB phototherapy combined with emollient and NB-UVB as monotherapy. By the end of 8 weeks, efficacy rates were 73.5% and 44.4%, while recurrence rates were 2.9% and 18.1%, respectively, suggesting that emollients are essential for patients with psoriasis.64
Corticosteroids
Topical corticosteroids are the most common topical treatment for psoriasis in the People’s Republic of China; they can be used as monotherapy or combined therapy. In the UK, topical corticosteroids and corticosteroid combination products are also frequently prescribed for psoriasis patients.65 They are the first line of therapy for mild and moderate psoriasis.66 Mild to mid-potency corticosteroids are commonly used for facial and flexural psoriasis, and high-potency corticosteroids are used for thick and intractable plaques. Occlusion of the medication can be used to increase the effect. Peng et al reported that 0.05% desonide cream was more effective than 1% hydrocortisone butyrate and was similar to 0.1% mometasone furoate cream.67 Another open-label study evaluated the efficacy of mometasone furoate in 190 subjects with plaque psoriasis. After 2 weeks, 54% of subjects attained a PASI score of 75.68 Compound steroids are also often used in the People’s Republic of China. Huang et al treated subjects with calcipotriol betamethasone ointment for 4 weeks; 73% of subjects reached a 75% reduction in PASI.69
Side effects of topical corticosteroids include skin atrophy, angiotelectasis, and secondary infection. Systemic absorption and side effects should be monitored when high-potency corticosteroids are used on a large area of skin for a long period of time. Tachyphylaxis has been reported.70 However, Miller et al reported that none of 32 subjects developed tachyphylaxis during 12 weeks of topical corticosteroid use.71 Tachyphylaxis can be the result of poor compliance rather than a decline in the binding ability to corticosteroid receptors.72
Vitamin D analogs
In the UK, topical vitamin D analogs are the second most used topical drugs for the treatment of psoriasis. About 39% of patients use topical vitamin D analogs.65 Vitamin D analogs include calcipotriol, tacalcitol, and calcitriol. They have similar effects as topical corticosteroid but with fewer side effects. A study by Ji et al evaluated the efficacy of calcitriol and calcipotriol ointments for plaque psoriasis. After 12 weeks of treatment, 91.1% of subjects in the calcitriol group and 81% in the calcipotriol group achieved good responses.73
However, topical vitamin D analogs can induce irritation in some patients. This usually occurs within a few days after initial use. A combination of topical vitamin D analogs and topical corticosteroids can enhance the effect and reduce the risk of this side effect. It can also help reduce the dosage of topical corticosteroid. Topical vitamin D analogs are widely used in the People’s Republic of China because they are steroid-free and have good clinical efficacy with minimal adverse effects in long-term use.
Retinoids
Topical retinoids such as tazarotene cream are also used in the treatment of psoriasis.66 However, a high incidence of skin irritation prevents their widespread use. To avoid this, they are often used in combination with topical corticosteroids. Zhang et al compared the efficacy and safety of an ointment containing tazarotene and 0.02% clobetasol ointment with tazarotene ointment monotherapy.74 After 8 weeks, 62% of subjects responded positively to the compound while only 38.1% responded to tazarotene ointment alone, indicating that the compound ointment was more effective. Another study also addressed the efficacy of tazarotene and calcipotriol in plaque psoriasis.75 Seventy-one subjects were randomized to tazarotene once daily in the evening or calcipotriol twice daily. After 12 weeks, 83% and 79% of subjects responded positively to tazarotene cream and calcipotriol ointment, respectively.
Calcineurin inhibitors
Tacrolimus is an effective treatment for facial and flexural psoriasis. A study by He et al compared the efficacy of 0.1% tacrolimus ointment and 5% pine tar ointment for facial and scalp psoriasis. Both drugs were used twice daily for 8 weeks. At the end of the 8 week period, successful treatment was achieved in 95% of subjects receiving tacrolimus ointment and 60% receiving pine tar ointment (P<0.05).76 Zhao et al conducted a self right–left comparative study of 29 subjects with plaque psoriasis. The subjects used 0.1% tacrolimus ointment on one side of their body and 0.005% calcipotriol ointment on the other side twice daily for 6 weeks. Tacrolimus ointment showed similar efficacy as calcipotriol ointment. Skin irritation occurred in three subjects with tacrolimus and disappeared soon after discontinuation.77 Zhong et al performed a study in 62 subjects with psoriasis to compare the efficacy and safety of 0.1% tacrolimus ointment and mometasone furoate ointment. After 4 weeks, PASI score decreased by 84% in the tacrolimus group compared to 70% in the mometasone furoate group, indicating that 0.1% tacrolimus ointment was more effective.78 A study by Li et al reported that 0.03% tacrolimus ointment in combination with and excimer laser treatment (308 nm) was more effective than tacrolimus alone.79
Phototherapy
Psoralen and ultraviolet A
PUVA is widely used in the People’s Republic of China. It is used to treat moderate and severe psoriasis, including widespread psoriasis vulgaris, pustular psoriasis, and erythrodermic psoriasis. Localized psoriasis plaques can be treated with PUVA as well. A study by Zhang et al compared the efficacy of PUVA with NB-UVB in the treatment of psoriasis vulgaris.80 Subjects received phototherapy three times weekly. When 95% reduction of lesions was achieved, the treatment was reduced to twice weekly, once weekly, and then once every 2 weeks for a total of 7 weeks. During treatment, subjects used only an emollient. PUVA showed similar efficacy as NB-UVB but subjects in the NB-UVB group experienced earlier response. However, discrepancies in results have been reported.81,82
UVB therapy
UVB therapy is standard therapy for plaque psoriasis and has been widely used in the People’s Republic of China for 40 years. Narrowband UVB (NB-UVB, 311 nm) has been used for psoriasis over the past 10 years and has been shown to be superior to conventional broadband (BB)-UVB. As a result, BB-UVB has been gradually replaced by NB-UVB. Li and Guo conducted a self-comparative study to evaluate BB-UVB and NB-UVB for plaque psoriasis. Fifty subjects received BB-UVB on the right side of their bodies and NB-UVB on the left side three times a week for 4 weeks. At end of treatment, clearance of psoriasis was observed in 90% of subjects in the NB-UVB group compared to 84% in the BB-UVB group, indicating that NB-UVB was more effective.83
NB-UVB is superior to BB-UVB with respect to both clearing and remission times.83 Treatment is administered 2–3 times weekly. Three times weekly has been shown to be more effective than twice weekly.84 Most subjects achieved a PASI of 75 after 20–30 treatments with NB-UVB therapy only.85,86 A retrospective study was conducted to compare NB-UVB monotherapy, acitretin monotherapy, and a combination of both for treatment of psoriasis vulgaris. After 26, 28, and 18 days, PASI score reductions were 78%, 79%, and 89% respectively, suggesting that NB-UVB monotherapy is as effective as acitretin and combination therapy is more effective than monotherapy.87Chen et al compared the efficacy of NB-UVB combined with methotrexate vs NB-UVB monotherapy in 69 subjects with psoriasis vulgaris.88 At 8 weeks, the effectiveness rate in the combined therapy group was 86% and 62.5% in the NB-UVB monotherapy group, indicating that NB-UVB and methotrexate had synergistic effects.
Antibiotics
Since infection is an important triggering factor for psoriasis in many patients, antibiotics are often used.61 They are effective for the treatment of psoriasis guttate, psoriasis vulgaris, pustular psoriasis, and erythrodermic psoriasis. Penicillin, cephalosporin, and erythromycin are among the most used antibiotics. Liu et al conducted a study to compare the effects of erythromycin and ampeptidum in subjects with psoriasis guttate. After 5 weeks, subjects receiving erythromycin achieved a 86% PASI reduction while subjects receiving ampeptidum had only a 58% reduction.89 In the People’s Republic of China, antibiotic therapy is a standard treatment for psoriasis guttate and acute exacerbations of plaque psoriasis.
Methotrexate
Methotrexate is effective for treating moderate to severe psoriasis vulgaris, psoriasis arthropathica, erythrodermic psoriasis, generalized pustular psoriasis, and psoriasis with severe impacts on quality of life such as palm and sole psoriasis. The initial dose of methotrexate is 5–15 mg a week. Li et al conducted an open-label study in 41 subjects with moderate to severe psoriasis. Subjects were administered methotrexate 5–15 mg weekly for 10–20 weeks; 80.5% of subjects were treated successfully.90 Zhang et al compared methotrexate, leflunomide, and a combination of low-dose methotrexate and leflunomide for psoriatic arthritis.91 By the end of 24 weeks of treatment, 75%, 31%, and 83% subjects achieved PASI 75 in the methotrexate, leflunomide, and combination groups, respectively, indicating that methotrexate was more effective than leflunomide and the combination of both can enhance efficacy. Methotrexate is well tolerated. There have been no reports of serious side effects regarding its use in psoriasis subjects in the People’s Republic of China. Common adverse effects are nausea, vomiting, and headache. Methotrexate should be discontinued if liver failure, leukopenia, or anemia develop.
Cyclosporin A
CsA is effective for all types of psoriasis. Indications for use are severe pustular psoriasis, psoriasis arthropathica, erythrodermic psoriasis, and widespread psoriasis vulgaris unresponsive to conventional treatment. The initial dose of CsA is 3–5 mg/kg/d, with a maintenance dose of 3 mg/kg/d. Most subjects can achieve clinical clearance within 4–8 weeks; a maintenance course of 1 year is recommended. Wang et al compared CsA with methotrexate for the treatment of severe psoriasis.92 CsA dose was 3–5 mg/kg/d and methotrexate dose was 10–15 mg weekly. By the end of 8 weeks, 84.6% of subjects in the CsA group and 79% in the methotrexate group showed significant improvements.
A French study showed that 50%–97% of subjects who received higher doses of cyclosporin (5 mg/kg/d) reached a PASI of 75 while only 28%–85% who received lower doses (2.5 mg/kg/d) reached PASI 75. In addition, the initial dose of 5 mg/kg/d led to a higher clearance rate. Eighty-nine percent and 50% of subjects with palmoplantar pustulosis and erythrodermic psoriasis obtained satisfactory effects after treatment with cyclosporin.93
Acitretin
Acitretin is a second generation systemic retinoid that has been approved for treating psoriasis for more than 10 years. Patients with generalized pustular and erythrodermic psoriasis can achieve satisfactory outcomes with acitretin treatment. Acitretin is also effective in psoriasis vulgaris and pustular psoriasis of the palms and soles. Acitretin-induced clearance of psoriasis is dose dependent. Higher starting doses seem to clear psoriasis faster.
The optimal initial dose of acitretin for treatment of psoriasis is 20–30 mg/d, with a maintenance dose of 20–50 mg/d. Most patients relapse within 2 months after discontinuation. In an open-label study by Yang and Chen, 23 subjects with severe psoriasis were given acitretin at an initial dose of 0.75–1.00 mg/kg/d and average maintenance doses of 10–30 mg/d. After 4–6 weeks, response rates were 87.5% in widespread plaque psoriasis, 83.3% in erythrodermic psoriasis, 100% in generalized pustular psoriasis, and 50% in psoriasis arthropathica.94 These results provide evidence that acitretin is effective in the treatment of severe psoriasis, especially generalized pustular psoriasis. Wang et al conducted a study involving 150 subjects with psoriasis vulgaris to compare the efficacy and safety of acitretin, NB-UVB, and combination therapy.95 After 4 weeks, response rates were 66.7%, 84.8%, and 98.4%, respectively. Combination therapy was more effective than monotherapy. In addition, doses of NB-UVB in combination therapy were lower than in monotherapy.
A French study by Sbidian et al showed that acitretin monotherapy appeared to provide better efficacy in pustular psoriasis than in psoriasis vulgaris.96 Combination of acitretin with phototherapy was more effective in subjects with psoriasis vulgaris. The recommended initial dose was 10–25 mg/d, and was gradually increased as required to reach higher clinical efficacy and fewer adverse events. It is essential to use acitretin with caution in child-bearing women. Individuals who take acitretin should avoid pregnancy until 2 years after drug discontinuation. Acitretin should be discontinued if liver failure, hyperlipidemia, or diffuse idiopathic hyperostosis develop.
Biologics
During the last decade, biologics have been explored in the treatment of psoriasis; some have been successful. Etanercept, a human recombinant TNF receptor p75 fusion protein, has been approved by the China Food and Drug Administration (CFDA) for treatment of psoriasis.61,97 Patients should meet the following criteria before being administered etanercept: 1) moderate or severe psoriasis with a PASI score >10 and a Dermatology Life Quality Index score >10; and 2) failure to respond to conventional systemic treatment.
A randomized controlled trial was conducted by Huang et al on 144 subjects with moderate to severe psoriasis vulgaris. Subjects were given subcutaneous etanercept 50 mg or oral methotrexate 7.5 mg once a week. By the end of 12 weeks, 76% of subjects in the etanercept group achieved PASI 75 and 53% achieved PASI 90, while in the methotrexate group, 44% achieved PASI 75 and 22% achieved PASI 90.98 In an open-label study by He et al, 20 subjects with generalized pustular psoriasis were treated with subcutaneous injections of etanercept 25 mg twice a week for two weeks, 12.5 mg twice a week for one week, and 12.5 mg once a week for one week.99 Twelve subjects were treated with prednisone 1 mg/kg/d in addition to traditional Chinese medicine. After 4 weeks, the response rate in the etanercept group was 85% compared to 25% in the prednisone group, indicating that etanercept was much more effective than prednisone. After discontinuation of etanercept, most subjects relapsed at various times. Repeat dosing with etanercept can achieve similar response rates.100
Clinical trials in the People’s Republic of China using other biologics such as infliximab, adalimumab, and ustekinumab for psoriasis have been completed and are awaiting CFDA approval. An important limitation for the use of biologics in the People’s Republic of China is that most biologics are not covered by medical insurance.
Conclusion
Psoriasis is a chronic recurrent skin disease that has a significant negative impact on patient quality of life. The prevalence of psoriasis in Chinese individuals is less than that in Caucasian populations. A cold and dry climate, bacterial infection, diet, and stress are important risk factors for development and exacerbation of psoriasis. Chinese patients with psoriasis can receive individualized treatment according to type and severity of the disease.
Disclosure
The authors reports no conflicts of interest in this work.
References
Ahlehoff O. Psoriasis and cardiovascular disease. Dan Med Bull. 2011;58(11):B4347. | |
Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. | |
Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. | |
Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69(6):1014–1024. | |
Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. | |
Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001;15(1):16–17. | |
Farber EM, Nail L. Epidemiology: natural history and genetics. In: Roenigk HR Jr, Maibach HI, editors. Psoriasis. 3rd ed. Marcel Dekker, New York: 1998:107–157. | |
Qian XC. Prevalence of psoriasis in Xinjiang region. J Xinjiang Med Univ. 1978;2:204. Chinese. | |
Psoriasis epidemiology study group. Report on epidemiological survey of psoriasis in China in 1984. J Dermatol Venereol. 1989;11(1):60–72. Chinese. | |
Xu YY, Tong ZC, Shen SF, et al. An epidemiological investigation on psoriasis in country dwellers of Suzhou area in Anhui Province. Acta Anhui Med Univ. 2001;36(6):483–485. Chinese. | |
Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–667. | |
Li MJ, Wang P, Cai M, et al. Prevalence and risk factors of psoriasis in Hainan province: an epidemiological survey. Chin J Dermatol. 2013;46(3):157–159. Chinese. | |
Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–456. | |
Fry L. Psoriasis. Br J Dermatol. 1988;119(4):445–461. | |
Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60(3):394–401. | |
Dogra S, Yadav S. Psoriasis in India: prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76(6):595–601. | |
Wei J, Liu M, Qu L, Xiao T, Cheng HD, He CD. Clinical and epidemiological study of 531 patients with psoriasis vulgaris in Liaoning region. Int J Dermatol Venereol. 2011;37(1):4–6. Chinese. | |
Kawada A, Tezuka T, Nakamizo Y, et al. A survey of psoriasis patients in Japan from 1982 to 2001. J Dermatol Sci. 2003;31(1):59–64. | |
Takahashi H, Takahashi I, Tsuji H, et al. Analysis of psoriatic patients registered in Asahikawa Medical College Hospital from 1983 to 2007. J Dematol. 2009;36(12):632–637. | |
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. | |
Baek HJ, Yoo CD, Shin KC, et al. Spondylitis is the most common pattern of psoriatic arthritis in Korea. Rheumatol Int. 2000;19(3):89–94. | |
Jamshidi F, Bouzari N, Seirafi H, Farnaghi F, Firooz A. The prevalence of psoriatic arthritis in psoriatic patients in Tehran, Iran. Arch Iran Med. 2008;11(2):162–165. | |
Kundakci N, Tursen U, Babiker MO, Gurgey E. The evaluation of the sociodemographic and clinical features of Turkish psoriasis patients. Int J Dermatol. 2002;41(4):220–224. | |
Grjibovski AM, Olsen AO, Magnus P, Harris JR. Psoriasis in Norwegian twins: contribution of genetic and environmental effects. J Eur Acad Dermatol Venereol. 2007;21(10):1337–1343. | |
Jesic S, Hosovski E. Prevalence of psoriasis vulgaris in West Serbia. Srp Arh Celok Lek. 1994;122(3–4):61–64. | |
Zhang XJ, Chen SY, Wang FX, et al. Analyses of genetic epidemiology of psoriasis vulgaris. Chin J Dermatol. 2000;33(6):383–385. Chinese. | |
Jiang YM. Analysis of the induct factor inquisition result of 364 cases psoriasis. J Clin Exp Med. 2006;5(3):215–216. Chinese. | |
Tan Pei Lin L, Kwek SK. Onset of psoriasis during therapy with fluoxetine. Gen Hosp Psychiatry. 2010;32(4):466. | |
Song R. A case of ampicillin induced psoriasis. Chin J Derm Venereol. 1996;10(1):59. Chinese. | |
Zhang GH. Analysis of 11 cases of chlorpromazine induced psoriasis. Sichuan Mental Health. 1997;10(4):275. Chinese. | |
Zhu CX, Ling YL. A case of procaine induced psoriasis. Chin J Derm Venereol. 1997;11(5):315. Chinese. | |
Zhu BY. A case of rabies vaccine induced psoriasis. Chin J Derm Venereol. 1999;13(2):116. Chinese. | |
Tian L, Huang ML, Gao Y. A case of hepatitis B vaccine induced psoriasis of newborn. J Dermatol Venereol. 2000;22(3):41–42. Chinese. | |
Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3(1):32–38. | |
Dika E, Varotti C, Bardazzi F, Maibach HI. Drug-induced psoriasis: an evidence-based overview and the introduction of psoriatic drug eruption probability score. Cutan Ocul Toxicol. 2006;25(1):1–11. | |
Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, Halevy S. Drug exposure and psoriasis vulgaris: case-control and case-crossover studies. Acta Derm Venereol. 2005;85(4):299–303. | |
Cohen AD, Kagen M, Friger M, Halevy S. Calcium channel blockers intake and psoriasis: a case-control study. Acta Derm Venereol. 2001; 81(5):347–349. | |
Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25(6):606–615. | |
Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study. Arch Intern Med. 2007;167(15):1670–1675. | |
Bryld LE, Sorensen TI, Andersen KK, Jemec GB, Baker JL. High body mass index in adolescent girls precedes psoriasis hospitalization. Acta Derm Venereol. 2010;90(5):488–493. | |
Zhang C, Zhu KJ, Zheng HF, et al. The effect of overweight and obesity on psoriasis patients in Chinese Han population: a hospital-based study. J Eur Acad Dermatol Venereol. 2011;25(1):87–91. | |
Cheng J, Yang XQ, Zhang L, Feng YJ, Liu F, Hui RS. Clinical features of 114 psoriasis vulgaris patients with abnormal body mass index. Medical Journal of Chinese People’s Liberation Army. 2009;34(3):252–253, 258. Chinese. | |
Naldi L, Addis A, Chimenti S, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology. 2008;217(4):365–373. | |
Di Lernia V, Tasin L, Pellicano R, Zumiani G, Albertini G. Impact of body mass index on retention rates of anti-TNF-alfa drugs in daily practice for psoriasis. J Dermatolog Treat. 2012;23(6):404–409. | |
Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88(5):1242–1247. | |
Setty AR, Curhan G, Choi HK. Smoking and the risk of psoriasis in women: Nurses’ Health Study II. Am J Med. 2007;120(11):953–959. | |
Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol. 2012;175(5):402–413. | |
Zhang XJ, Wang FX, Yang S, et al. Analysis of the relationship between smoking, alcoholism and psoriasis. Chin J Derm Venereol. 2000;14(4):221–222. Chinese. | |
Verhoeven EW, Kraaimaat FW, Jong EM, Schalkwijk J, van de Kerkhof PC, Evers AW. Effect of daily stressors on psoriasis: a prospective study. J Invest Dermatol. 2009;129(8):2075–2077. | |
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139(6):752–756. | |
Zhang GW, Shao CG, Wang GC. The annual incidence and susceptible rate of age in China. J Dermatol Venereol. 1990;(1):62–68. Chinese. | |
Jacobson CC, Kumar S, Kimball AB. Latitude and psoriasis prevalence. J Am Acad Dermatol. 2011;65(4):870–873. | |
Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr. 2013;16(3):143–152. | |
Wei SP, Si RL, Zhang XG, et al. Logistic regression analysis on risk factors of psoriasis. Chin J Dermatol. 2004;37(11):665–666. Chinese. | |
Tong ZC, Xu YY, Shen SF, et al. Logistic regression analysis on risk factors of psoriasis. Chin J Dis Control Prev. 2002;6(4):319–320. Chinese. | |
Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128(1):39–42. | |
Wu W, Debbaneh M, Moslehi H, Koo J, Liao W. Tonsillectomy as a treatment for psoriasis: a review. J Dermatolog Treat. 2014;25(6):482–486. | |
Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–1565. | |
Wu Y, Lin Y, Liu HJ, Huang CZ, Feng AP, Li JW. Childhood psoriasis: a study of 137 cases from central China. World J Pediatr. 2010;6(3):260–264. | |
Chiam LY, de Jager ME, Giam YC, de Jong EM, van de Kerkhof PC, Seyger MM. Juvenile psoriasis in European and Asian children: similarities and differences. Br J Dermatol. 2011;164(5):1101–1103. | |
Psoriasis working group of Chinese society of Dermatology. Guidelines for management of psoriasis 2008. Chin J Dermatol. 2009;42(3):213–214. Chinese. | |
Hu JW. Clinical observation of tacalcitol combined with emollient in the treatment of psoriasis vulgaris. Chinese Manipulation and Rehabilitation Medicine. 2011;2(1):11–12. Chinese. | |
Qi L, Liu LF. 30 cases of psoriasis vulgaris treated with emollient. Henan Traditional Chinese Medicine. 2010;30(11):1099–1100. Chinese. | |
Wang HB, He L, Chen SH. Narrow band UVB combined with emollient in the treatment of psoriasis vulgaris. J Dermatol Venereol. 2013;35(1):29–30. Chinese. | |
Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. | |
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. | |
Peng ZH, Pan M, Ren JW. Comparison of the efficacy and safety of 0.05% desonide cream in patients with three common dermatoses. Chin J Derm Venereol. 2009;23(1):63–64. Chinese. | |
Ji SZ, Yang HZ, Li LF, et al. Treatment of plaque psoriasis with momerasonefuroate: a multicenter, open-label trial. J ClinDermatol. 2004;33(4):242–243. Chinese. | |
Huang L, Ma L, Huang Q, et al. Calcipotriol betamethasone ointment in the treatment of psoriasis vulgaris: a randomized, double-blind, active-control, parallel group study. Chin J Dermatol. 2009;42(10):691–694. Chinese. | |
du Vivier A, Stoughton RB. Tachyphylaxis to action of topically applied corticosteroid. Arch Dermatol. 1975;111(5):581–583. | |
Miller JJ, Roling D, Margolis D, Guzzo C. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41(4):546–549. | |
Feldman SR. Tachyphylaxis to topical corticosteroids: the more you use them, the less they work? Clin Dermatol. 2006;24(3):229–230. | |
Ji SZ, Chen XX, Wang BX, et al. Calcitriol ointment versus calcipotriol ointment in the treatment of psoriasis: a single-blind, randomized, multicenter trial. Chin J Dermatol. 2008;41(3):153–156. Chinese. | |
Zhang J, Diao QC, Yan GF, Zhu XJ. Observation of efficacy and safety of tazarotene ointment in the treatment of plaque psoriasis. Chong Qing Yi Xue. 2008;37(12):1293–1294. Chinese. | |
Li H, Li GM, Zhou X, et al. The efficacy of tazarotene and calcipotriol in the treatment of plaque psoriasis. Chin J New Drugs Clin Rem. 2002;21(3):155–158. Chinese. | |
He YL, Guo ZP. Clinical efficacy of 0.1% tarcrolimus ointment on plaque psoriasis of scalp and face. J Clin Dermatol. 2008;37(4):254–255. Chinese. | |
Zhao N, Jin HZ. Efficacy and safety of tacrolimus ointment and calcipotriol ointment in the treatment of plaque psoriasis. J Clin Dermatol. 2012;41(10):626–628. Chinese. | |
Zhong CM, Sun LD, Zeng H, Zhou ZG, He FJ. A clinical trial of 0.1% tacrolimus in the treatment of psoriasis vulgaris. J Clin Dermatol. 2010;39(8):531–532. Chinese. | |
Li W, Wang LX, Liu Y, Zhang XB. Clinical efficacy of 0.03% topical tacrolimus ointment combined with 308 nm excimer laser in treatment of psoriasis vulgaris. Chin J Derm Venereol. 2012;26(12):1152–1154. Chinese. | |
Zhang TZ, Zhou FH, Huang XR, Zhang Q, Liu XL, Zhou XY. Analysis of clinical efficacy of PUVA and narrow band ultraviolet B in the treatment of psoriasis vulgaris. J Clin Dermatol. 2006;35(2):111–112. Chinese. | |
Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol. 1999;41(5 Pt 1):728–732. | |
Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142(7):836–842. | |
Li JY, Guo ZP. Analysis of clinical efficacy of 311 nm narrow band UVB and 280–320 nm broad band UVB phototherapy in chronic plaque psoriasis. J Clin Dermatol. 2006;35(3):185–187. Chinese. | |
Cameron H, Dawe RS, Yule S, Murphy J, Ibbotson SH, Ferguson J. A randomized, observer-blinded trial of twice vs three times weekly narrow-band ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147(5):973–978. | |
Li YT, Shi TX, Qin ZF. Clinical study on the treatment of psoriasis with narrow-band UVB. J Clin Dermatol. 2012;41(12):763–764. Chinese. | |
Liu ZR, Gao TW, Li TH, et al. Observation of the efficacy of 311 nm narrow-band ultraviolet B phototherapy in psoriasis vulgaris. J Clin Dermatol. 2004;33(6):373–375. Chinese. | |
Wang DY, Peng ZH, Ge WY, Liu P, Wang YX, Tan SS. Observation of the efficacy of narrow-band ultraviolet B phototherapy combined with acitretin on plaque psoriasis. Chin J Derm Venereol. 2005;19(8):477–478. Chinese. | |
Chen ZX, Ni WQ, Wei F. Efficacy of narrow-band ultraviolet B phototherapy combined with methotrexate on psoriasis vulgaris. J Clin Dermatol. 2007;36(9):596–597. Chinese. | |
Liu W, Zhao G. Efficacy of erythromycin in the treatment of psoriasis guttata. J Clin Dermatol. 2005;34(8):551. Chinese. | |
Li CX, Zhang XB, Huang ZM, Liu YM, Wu ZH, Chen QX. Methotrexate in the treatment of patients with moderate to severe psoriasis. J Clin Dermatol. 2006;35(5):327–328. Chinese. | |
Zhang GL, Huang F, Zhang JL, Li XF, Zhang LY, Hao HQ. A clinical study on leflunomide and methotrexate for skin involvement of psoriatic arthritis. Chin J Rheumatol. 2010;14(4):256–259. Chinese. | |
Wang S, Ai DF, Li SL, Wang Q, Wang XY. The efficacy of cyclosporine A and methotrexate in treatment of severe psoriasis. Chin J Lepr Skin Dis. 2012;28(8):554–555. Chinese. | |
Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporine in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25 Suppl 2:19–27. | |
Yang XY, Chen ZQ. Evaluation on the effectiveness of acitretin in severe psoriasis. J Clin Dermatol. 2003;32(10):610–611. Chinese. | |
Wang SF, Song SN, Liu XL, Wang YP. Therapeutic effect of narrow-band UVB in combination with acitretin in treating 150 cases of psoriasis. Chin J Derm Venereol. 2008;22(3):192–193. Chinese. | |
Sbidian E, Maza A, Montaudie H, et al. Efficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature review. J Eur Acad Dermatol Venereol. 2011;25 Suppl 2:28–33. | |
Smith CH, Anstey AV, Barker JN, et al. British association of dermatologists guidelines for use of biological interventions in psoriasis 2005. Br J Dermatol. 2005;153(3):486–497. | |
Huang Q, Yang QP, Fang X, et al. Treatment of psoriasis vulgaris with a recombinant human tumor necrosis factor receptor: Fc fusion protein: a multicenter, randomized, double blind trial. Chin J Dermatol. 2007;40(11):655–658. Chinese. | |
He QB, Yin GW, Li HW, Dong HT. The observation of efficacy of entanercept in treatment of 20 cases of generalized pustular psoriasis. J Zhengzhou Univ Med. 2010;45(4):668–670. Chinese. | |
Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
