Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Emerging treatments in type 2 diabetes: focus on canagliflozin
Authors Rosiak M, Grzeszczak S, Kosior D, Postuła M
Received 22 December 2013
Accepted for publication 1 April 2014
Published 21 August 2014 Volume 2014:10 Pages 683—689
DOI https://doi.org/10.2147/TCRM.S39145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Marek Rosiak,1,2 Susanna Grzeszczak,2 Dariusz A Kosior,2,3 Marek Postuła1,2
1Department of Cardiology and Hypertension, Central Clinical Hospital, the Ministry of the Interior, Warsaw, Poland; 2Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Poland; 3Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland
Abstract: Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder, which affects more than 300 million people globally. The common effect of uncontrolled diabetes is the state of hyperglycemia, which results from beta-cell dysfunction as well as insulin resistance, which is accompanied with microvascular and macrovascular complications. As hyperglycemia defines diabetes, glycemic control is fundamental to the management of diabetes. Sodium glucose co-transporter 2 inhibitors (SGLT2) are a new group of oral antidiabetic medications that act by blocking the reabsorption of glucose, causing it to be excreted in the urine. Canagliflozin was the first SGLT2 inhibitor to be approved in the US by the Food and Drug Administration for the treatment and control of T2DM and on September 19, 2013, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Invokana®. Canagliflozin is a SGLT2 inhibitor, which acts upon the proximal tubules of the kidneys and reduces the renal threshold for glucose. It is highly selective, binding 250 times more potently to SGLT2 than sodium glucose co-transporter 1 inhibitor. This action allows a higher amount of glucose to be excreted within the urine, causing the patient's plasma glucose level to be decreased and indirectly causing weight loss. Among the most common adverse events are hypoglycemia, headache, nausea, female genital and urinary tract infections, nasopharyngitis, and transient postural dizziness. Given its high efficacy in reducing hyperglycemia and good safety profile as either monotherapy or an add-on treatment to metformin, sulfonylureas, or insulin, canagliflozin seems to be a promising antihyperglycemic drug. Nevertheless, further large-scale and long-term studies should be conducted to evaluate the impact of canagliflozin on cardiovascular risk in T2DM patients.
Keywords: hyperglycemia, SGLT2 inhibitor, hypertension, dapagliflozin, insulin resistance, obesity, blood pressure, weight loss
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder that affects more than 300 million people globally.1 The common effect of uncontrolled diabetes is the state of hyperglycemia, which results from beta-cell dysfunction as well as insulin resistance, which is accompanied by microvascular and macrovascular complications.2 As hyperglycemia defines diabetes, glycemic control is fundamental to the management of diabetes. Current oral antihyperglycemic drugs, prescribed alongside diet and exercise modification, include oral antihyperglycemic drugs (ie, metformin, sulfonylureas [SU], alpha-glucosidase inhibitors, meglitinides, and dipeptidyl peptidase-4 [DPP-4] inhibitors), and injectable medications, including insulins, incretin mimetics, and amylin analogs. Nonetheless, at least 50% of patients cannot achieve proper control of their glycemia; consequently, an innovative pharmacological treatment is required to aid diabetic patients.3
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a new group of oral antidiabetic medications that act by blocking the reabsorption of glucose, causing it to be excreted in the urine.4,5 Canagliflozin was the first SGLT2 inhibitor to be approved in the US for the treatment and control of T2DM, which occurred in March 2013 by the US Food and Drug Administration (FDA) and on September 19, 2013, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Invokana® (Janssen Pharmaceuticals, Johnson & Johnson, New Brunswick, NJ, USA), film-coated tablets containing 100 mg and 300 mg of canagliflozin.6
Pharmacodynamics
The kidneys aid in the regulation of plasma glucose levels, with over 180 L of plasma maintaining a glucose concentration of 90 mg/dL, filtered by the glomeruli over 24 hours. Within healthy patients, all of the glucose is reabsorbed by the proximal tubules, via SGLT2. The maximum glucose transport capacity of the proximal tubules is nearly 375 mg/minute. Diabetics exceed this capacity, termed the “renal threshold”, accounting for the presence of glucose within their urine.7 SGLT2 transporters have a low affinity for glucose (Km =3 mM), have a high transport capacity (Tmax =10 nmol/mg protein × minutes), and allow for the transportation of one glucose molecule for every sodium ion.8 Canagliflozin is a SGLT2 inhibitor, which acts upon the proximal tubules of the kidneys and reduces the renal threshold for glucose. It is highly selective, binding 250 times more potently to SGLT2 than sodium glucose co-transporter 1 (SGLT1). This action allows a higher amount of glucose to be excreted with the urine, causing the patient’s plasma glucose level to be decreased and indirectly causing weight loss.3 The 50% inhibitory concentrations (IC50) are 2.2–4.4 nmol/L and 684–910 nmol/L for SGLT2 and SGLT1, respectively. Dose-dependent decreases in renal threshold for glucose and increases in urinary glucose excretion (UGE) were observed when single and multiple oral doses were administered to T2DM patients. In a study by Devineni et al, canagliflozin increased UGE in a dose-dependent manner compared to placebo (changes of UGE in 27-day observation for 100 mg and 300 mg of canagliflozin and placebo were −3.2, 71.9, and 129.2 g/day, respectively).9 Decreases in plasma glucose in a dose-dependent fashion were also noted as early as the first day of administration, with similar reductions occurring on the last day of treatment. When given to healthy and T2DM patients before a meal, a delay in intestinal glucose absorption and a reduction in postprandial glucose was observed.10 High concentrations of canagliflozin within the proximal lumen of the gut may lead to temporary SGLT1 inhibition, which is assessed via a hydrogen breath test.9
Pharmacokinetics
The pharmacokinetics of canagliflozin are similar in healthy subjects and patients with T2DM.11 Canagliflozin is swiftly absorbed across a wide dose range in a dose-dependent manner, from 50–300 mg (once daily) and accumulation in plasma has been observed following multiple doses of 100–300 mg.12 As such, the time to reach half-life elimination and maximal concentration within the plasma (Cmax) are independent of the dose. The time to reach steady state in a once daily dose of 100–300 mg is 4–5 days. Based on its potential to reduce postprandial plasma glucose (PPG) excursions due to delayed intestinal glucose absorption, it is recommended that canagliflozin is taken before the first meal of the day.11 The oral bioavailability of canagliflozin is approximately 65%, with almost 99% bound to plasma proteins, especially albumin. Protein binding is independent of canagliflozin plasma concentrations and is not significantly altered in patients with renal or hepatic impairment. The main metabolic pathway of canagliflozin is that of O-glucuronidation, which causes the breakdown of canagliflozin into O-glucuronides, two inactive products. The enzymes that facilitate this process are UGT1A9 and UGT2B4. To a lesser extent (7%), canagliflozin also undergoes oxidative metabolism via CYP3A4. Canagliflozin weakly inhibits CYP2B6, CYP2C8, CYP2C9, and CYP3A4. Approximately 33% of canagliflozin metabolites are renally eliminated and approximately 42% are excreted in the feces, about 1% of which remains unchanged. Furthermore, canagliflozin was found to not be carcinogenic, mutagenic, or affecting fertility.3
Sex, age, body weight, and race do not alter the pharmacokinetics of canagliflozin to a clinically relevant extent.11 Renal impairment does not affect the Cmax of canagliflozin and there were no clinically meaningful changes in the area under the plasma concentration–time curve (AUC) values in patients with impaired renal function. However, the pharmacodynamics response to canagliflozin declines with increasing severity of renal impairment, with a maximum recommended dosage of 100 mg once daily in those with moderate renal impairment (estimated glomerular filtration rate [eGFR]: 45–60 mL/minute/1.73 m2). Canagliflozin is not recommended in patients with an eGFR of ≤45 mL/minute/1.73 m2 and is negligibly removed by hemodialysis.11
Yale et al conducted a study comparing the efficacy and safety of both doses of canagliflozin with placebo in patients with stage 3 chronic kidney disease (CKD; eGFR ≥30 and <50 mL/minute/1.73 m2). During 26 weeks of treatment, a transient decrease in eGFR that recovered towards baseline over the study period was seen in all groups, but was a little higher in patients treated with 100 mg and 300 mg of canagliflozin than in the placebo group (−9.1%, −10.1%, and −4.5%, respectively). There was also a marked increase in urea nitrogen concentration in studied groups, which was slightly higher in the patients taking 100 mg and 300 mg of canagliflozin compared to placebo (12.1%, 12.5%, and 4.9%, respectively). The results of the study suggest that canagliflozin may be an appropriate treatment option for patients with T2DM and stage 3 CKD.12
Mild and moderate hepatic impairment have no clinically meaningful effect on the pharmacokinetics of canagliflozin.11 Currently, there are no data of efficacy and safety of canagliflozin in patients with severe liver failure.
Side effects and safety
Canagliflozin is generally a well-tolerated oral medication, with most reported adverse events (AEs) being mild and not life-threatening (Table 1). Canagliflozin reduces plasma glucose concentration via an insulin-independent mechanism; thus, the incidence of hypoglycemia observed in clinical trials was 3.0%–5.1% with no reported cases of discontinuation due to severe hypoglycemia. In the CANTATA-SU (Canagliflozin Treatment and Trial Analysis – Sulfonylurea) trial, the incidence of hypoglycemia was lower in canagliflozin than in glimepiride-treated patients (5%–6% versus [vs] 34%, respectively).13
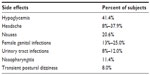  | Table 1 Side effects of canagliflozin reported in clinical trials |
Blocking SGLT2 decreases renal reabsorption of glucose and increases urine glucose concentration. This consequently leads to increased rates of genital mycotic infections and urinary tract infections (UTIs). In study conducted by Nyirjesy et al, treatment with canagliflozin compared to sitagliptin increased vaginal colonization with Candida species (31% vs 14%, respectively; odds ratio [OR]: 2.8; 95% confidence interval [CI]: 1.0–7.3), and symptomatic vulvovaginal adverse events (10% vs 3%, respectively; OR: 9.1; 95% CI: 2.4–34.0) in women with T2DM. In patients treated with canagliflozin, there was also a higher rate of UTIs than in the control group (5.0% vs 3.8%, respectively; OR: 1.31; 95% CI: 0.45–4.68).14 Other AEs during canagliflozin treatment included nasopharyngitis and transient postural dizziness.
Clinical trials on canagliflozin
Completed trials
Canagliflozin has been studied in several trials comparing its safety and efficacy as monotherapy or additional therapy with placebo, metformin, SU, and pioglitazone (Table 2). Recently the results of several randomized, double-blind clinical trials grouped under one acronym CANTATA (Canagliflozin Treatment and Trial Analysis) have been released. The aim of these studies was to compare two doses of canagliflozin with other antidiabetic treatment strategies in terms of their efficacy (change from baseline glycated hemoglobin [HbA1c] at 26 weeks or 52 weeks) and safety.15
  | Table 2 Endpoints of various trials concerning canagliflozin |
CANTATA-M (Canagliflozin Treatment and Trial Analysis – monotherapy), a randomized, double-blind, placebo-controlled study included both patients with inadequate glycemic control on diet and exercise, and patients on antihyperglycemic treatment who had to undergo an 8-week antihyperglycemic agents washout period with diet and exercise. All patients entered into a 2-week, single-blind, placebo run-in period prior to randomization into a 26-week, double-blind, placebo-controlled core treatment period. Patients were randomly assigned in a 1:1:1 ratio to once-daily oral doses of canagliflozin 100 mg or 300 mg, or matching placebo, at entry into the core period. After completing the core study period, patients then entered a 26-week, double-blind, active-controlled extension period. Patients receiving canagliflozin 100 mg or 300 mg continued treatment, while patients who had been taking placebo during the core period switched in a blinded fashion to double-blind, active treatment with sitagliptin 100 mg upon entering the extension period.16
In this trial, the impact of 100 mg or 300 mg of canagliflozin in monotherapy versus placebo on HbA1c, fasting plasma glucose (FPG), 2-hour PPG, systolic blood pressure (SBP), change in triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) concentration, as well as change in body weight have been evaluated. Patients treated with the canagliflozin doses (100 mg and 300 mg) produced a significant (P<0.001 for both canagliflozin doses) decrease in HbA1c (−0.91% and −1.16%, respectively), FPG (−2 mmol/L and −2.4 mmol/L, respectively), and 2-hour PPG (−2.7 mmol/L and −3.6 mmol/L, respectively) at week 26. Moreover, significant decreases in body weight (−1.9 kg and −2.9 kg) and increases in HDL-C concentrations (6.8% and 6.1%) were observed (P<0.001 for both doses of canagliflozin).16
Recently, the results of the second, active phase of CANTATA-M trial have been demonstrated at week 52: canagliflozin 100 mg and 300 mg provided dose-related decreases from baseline in HbA1c of −0.81% and −1.11%, respectively. Canagliflozin 100 mg and 300 mg respectively decreased FPG (−1.5 mmol/L and −2.2 mmol/L [−27.4 and −39.1 mg/dL]), body weight (−3.3% and −4.4%), and systolic BP (−1.4 and −3.9 mmHg), and decreased TGs and increased HDL-C and LDL-C.17
The overall incidences of AEs over 52 weeks were 67.2%, 66.0%, and 64.1%, and rates of serious AEs were 5.6%, 2.5%, and 5.7%, with canagliflozin 100 mg and 300 mg and placebo/sitagliptin, respectively. The incidences of documented hypoglycemia were 5.1%, 3.6%, and 3.6%, respectively, with no events leading to discontinuation. Over 52 weeks, the incidence of genital mycotic infections was higher with canagliflozin than with placebo/sitagliptin in females and males. The incidences of UTIs were 8.2%, 7.1%, and 6.3% with canagliflozin 100 mg and 300 mg and placebo/sitagliptin, respectively; there were no serious events or incidences of upper UTIs (ie, pyelonephritis), and no UTI AEs led to study discontinuation.17
Similar results have been demonstrated in the CANTATA-MP (Canagliflozin Treatment and Trial Analysis – Metformin and Pioglitazone) trial comparing both canagliflozin doses (100 mg and 300 mg) with placebo in patients with T2DM treated with metformin and pioglitazone and with baseline HbA1c at 7%–10.5%. At week 26, a significant (P<0.001 for both doses of canagliflozin compared to placebo) decrease in HbA1c (−0.62% and −0.76%), FPG (−29 mg/dL and −36 mg/dL), and body weight (−2.7% and −3.7%) was observed.18
In CANTATA-SU, patients with T2DM inadequately controlled with metformin (HbA1c at 7.0%–9.5%) were randomly assigned in a 1:1:1 ratio to 100 mg or 300 mg of canagliflozin or glimepiride. For lowering of HbA1c at 52 weeks, canagliflozin 100 mg was non-inferior to glimepiride (least squares mean difference: −0.01% [95% CI: −0.11 to 0.09]), and canagliflozin 300 mg was superior to glimepiride (−0.12% [95% CI: −0.22 to −0.02]). More patients treated with glimepiride had serious adverse events than patients treated with both doses of canagliflozin (8% vs 5% in both canagliflozin doses, respectively). Nevertheless, in 100 mg and 300 mg canagliflozin groups compared to glimepiride, there were significantly higher rates of mycotic infections (11% and 14% vs 2% in women; 7% and 8% vs 1% in men, respectively), UTIs (6% for both canagliflozin doses vs 5%), and osmotic diuresis-related events (pollakiuria: twelve [3%] for both doses vs one [<1%]; polyuria: four [<1%] for both doses vs two [<1%]).13
Canagliflozin was also studied as a therapy adjunctive to insulin in an 18-week, double-blind, placebo-controlled trial in a subset of the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial. Patients were enrolled in the study if their diabetes was not well-controlled on insulin, on ≥30 units of insulin per day, or on insulin with oral antihyperglycemic medications. Canagliflozin (100 mg and 300 mg) reduced FPG (by 25–31 mg/dL) and body weight (by 1.8%–2.7%) versus the placebo group. Also, most dose reductions of insulin were made in the canagliflozin group.19
The incidence of AEs leading to discontinuation was greater with canagliflozin 300 mg than with canagliflozin 100 mg or placebo (5.3%, 1.9%, and 1.9%, respectively). Adverse events of genital mycotic infections in men and women, increased urination (pollakiuria), and hypotension were more common with canagliflozin 100 mg and 300 mg compared to placebo in men and women; these specific adverse events were generally mild or moderate in intensity and infrequently led to discontinuation. A slightly higher incidence of UTI was seen with canagliflozin 300 mg than canagliflozin 100 mg or placebo. The incidence of hypoglycemia was higher with canagliflozin 100 mg and 300 mg than placebo (49% and 48% vs 37%, respectively). Overall, the adverse events that resulted in discontinuation of treatment or serious AEs were higher in the canagliflozin group compared to placebo. In addition, the proportion of subjects who underwent a hypoglycemic state during the trial (≤70 mg/dL or severe events) was higher in the 100 mg and 300 mg canagliflozin groups compared to placebo (42% and 43% vs 25%, respectively). In conclusion, canagliflozin used as an adjunctive therapy to basal insulin improved glycemic control and reduced body weight. It was also well-tolerated.19
Ongoing trials
In June 2013, an interventional, randomized, 26-week, Phase III trial started (NCT01809327). In a group of 1,180 patients, the effectiveness (defined as change in HbA1c) of coadministering two doses of canagliflozin (100 mg or 300 mg) with metformin extended release (XR) in comparison to 100 mg or 300 mg of canagliflozin alone, and metformin XR alone within inadequately controlled T2DM patients is being assessed. The secondary outcome measures include changes in body weight, changes in the percentage of participants with well-controlled diabetes (defined as HbA1c <7% after follow-up period), changes in HDL-C and TG concentrations, and changes in SBP. The safety and tolerability of canagliflozin will also be weighed. Study completion is planned for December 2014.20
The purpose of another study is to evaluate in a 6-week follow-up period the effect of two doses of canagliflozin (100 mg or 300 mg daily) on blood pressure reduction, compared to placebo, in approximately 189 patients with hypertension and T2DM, who are on stable doses of antihyperglycemic and antihypertensive agents (NCT01939496). The estimated study completion date is December 2014.21
A double-blind, interventional, Phase I trial that commenced in June 2013 will compare the pharmacodynamics of canagliflozin with the pharmacodynamics of dapagliflozin. The primary objective of this study will be the comparison of a 24-hour mean renal threshold for glucose for canagliflozin (300 mg once daily for 4 days) compared to dapagliflozin (10 mg once daily for 4 days) throughout a 4-day time period (NCT01877889).22
Lastly, a planned interventional Phase IV trial started recruiting participants in September 2013 to gauge the effect of canagliflozin on blood pressure reduction versus a placebo, in patients with hypertension and T2DM and who are on stable doses of antihyperglycemic and antihypertensive agents. Furthermore, the overall safety and tolerability of canagliflozin will be assessed. The main objective of this study is to observe the changes within the mean 24-hour SBP by ambulatory blood pressure monitoring (ABPM) throughout a 6-week period (NCT01939496).23
The role of SGLT-2 inhibitors in current guidelines
Due to the very few clinical data regarding the long-term efficacy and safety of SGLT2 inhibitors, the International Diabetes Federation (IDF) guidelines for managing older people with T2DM recommend considering this group of drugs as an alternative therapy to metformin, SU, or DDP-4 inhibitors as a first- (in monotherapy), second-, or third-line therapy (dual or triple therapy).24 Also, the National Institute for Health and Care Excellence (NICE) guidelines recommend the use of dapagliflozin in a dual therapy regimen in combination with metformin or with insulin with or without other antidiabetic medication. The authors do not recommend combining dapagliflozin with metformin and SU as an option for treating T2DM except as part of clinical trials.25 In the American Association of Clinical Endocrinologists’ Comprehensive Diabetes Management Algorithm 2013, the authors recommend that if weight loss is a therapeutic goal, then metformin plus a SGLT-2 inhibitor along with intensive lifestyle management would be preferable to other therapies that are weight promoting.26
Conclusion
Canagliflozin is a well-tolerated, novel treatment for patients with poorly controlled T2DM. It inhibits a SGLT2 receptor in the proximal tubules of kidneys and reduces the renal threshold for glucose.12 This results in substantial reduction in HbA1c (−1.16%) and FPG (−2.4 mmol/L), as well as decreasing body weight and SBP during canagliflozin therapy. Canagliflozin is characterized by relatively low AE rates, including hypoglycemia, but it seems to increase the risk of genital mycotic infections and UTIs.9 Canagliflozin treatment requires dose reduction in patients with mild renal impairment (eGFR between 45–60 mL/minute/1.73 m2) but it should not be used in patients with eGFR ≤45 mL/minute/1.73 m2. Given its high efficacy in reducing hyperglycemia and good safety profile as either monotherapy or an add-on treatment to metformin, SU, or insulin, canagliflozin seems to be a promising antihyperglycemic drug.10 Nevertheless, further large-scale and long-term studies should be conducted to evaluate the impact of canagliflozin on cardiovascular risk in T2DM patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. | |
Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32(2):63–71. | |
Elkinson S, Scott L. Canagliflozin: first global approval. Drugs. 2013;73(9):979–988. | |
Triplitt CL. Understanding the kidneys’ role in blood glucose regulation. Am J Manag Care. 2012;18(Suppl 1):S11–S16. | |
INVOKANA™ [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013. | |
Committee for Medicinal Products for Human Use of the European Medicines Agency. EMA/374133/2013. Assessment report on the use of canagliflozin. London, UK: European Medicines Agency; 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002649/WC500156457.pdf. Accessed April 2, 2014. | |
Turk E, Martin MG, Wright EM. Structure of the human Na+/glucose cotransporter gene SGLT1. J Biol Chem. 1994;269(21):15204–15209. | |
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reasbsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1):397–404. | |
Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14(6):539–545. | |
Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13(7):669–672. | |
Janssen Pharmaceuticals Inc. Invokana™ (canagliflozin) tablets for oral use: US prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013. Available from: http://www.janssenmd.com/pdf/invokana/PI-INVOKANA.pdf. Accessed September 1, 2013. | |
Yale J, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–473. | |
Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. | |
Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28(7):1173–1178. | |
Nisly S, Kolanczyk D, Walton A. Canagliflozin, a new sodium-glucose cotransporter 2 inhibitor, in treatment of diabetes. Am J Health Syst Pharm. 2013;70(4):311–319. | |
Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–382. | |
Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2013;15(4):372–382. | |
Janssen Research and Development, LLC. The CANTATA-MP Trial (CANagliflozin Treatment and Trial Analysis – Metformin and Pioglitazone). Available from: http://clinicaltrials.gov/ct2/show/NCT01106690. NLM identifier: NCT01106690. Accessed September 20, 2013. | |
Janssen Research and Development, LLC [homepage on the Internet]. First Results from Phase 3 CANVAS Trial Show Canagliflozin as Add-on Therapy to Insulin Lowered Blood Sugar Levels in Patients With Type 2 Diabetes at an Elevated Risk for Cardiovascular Disease [October 2, 2012]. Titusville, NJ: Janssen Pharmaceuticals, Inc. Available from: http://www.investor.jnj.com/releasedetail.cfm?releaseid=710584. Accessed October 23, 2013. | |
Janssen Research and Development, LLC. A Study to Evaluate the Effectiveness, Safety, and Tolerability of Canagliflozin in Combination With Metformin in the Treatment of Patients With Type 2 Diabetes Mellitus With Inadequate Glycemic Control With Diet and Exercise. Available from: http://clinicaltrials.gov/ct2/show/NCT01809327. NLM identifier: NCT01809327. Accessed September 20, 2013. | |
Janssen Scientific Affairs, LLC. Evaluation of Blood Pressure Reduction, Safety, and Tolerability of Canagliflozin in Patients With Hypertension and Type 2 Diabetes Mellitus on Stable Doses of Anti-hyperglycemic and Anti-hypertensive Agents. Available from: http://clinicaltrials.gov/ct2/show/NCT01939496. NLM identifier: NCT01939496. Accessed September 20, 2013. | |
Janssen-Cilag International NV. A Study to Compare the Pharmacodynamics of Canagliflozin and Dapagliflozin in Healthy Volunteers. Available from: http://clinicaltrials.gov/ct2/show/NCT01877889. NLM identifier: NCT01877889. Accessed September 20, 2013. | |
Janssen Scientific Affairs, LLC. Evaluation of Blood Pressure Reduction, Safety, and Tolerability of Canagliflozin in Patients With Hypertension and Type 2 Diabetes Mellitus on Stable Doses of Anti-hyperglycemic and Anti-hypertensive Agents. Available from: http://clinicaltrials.gov/ct2/show/NCT01939496. NLM identifier: NCT01939496. Accessed September 20, 2013. | |
Taylor S, Harris K. The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy. 2013;33(9):984–999. | |
International Diabetes Federation. Global Guideline for Managing Older People with Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation; 2013. Available from: http://www.idf.org/sites/default/files/IDF-Guideline-for-older-people-T2D.pdf. Accessed April 2, 2014. | |
National Institute for Health and Care Excellence. Dapagliflozin in combination therapy for treating type 2 diabetes. Manchester, UK: National Institute for Health and Care Excellence; 2013. Available from: http://www.nice.org.uk/nicemedia/live/14193/64270/64270.pdf. Accessed April 2, 2014. | |
Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12 week study. Diabetes Obes Metab. 2013;15(12):1136–1145. | |
Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35(6):1232–1238. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
