Back to Journals » Clinical Ophthalmology » Volume 8
Effect of intravitreal bevacizumab on diabetic macular edema with hard exudates
Received 17 April 2014
Accepted for publication 28 May 2014
Published 12 August 2014 Volume 2014:8 Pages 1479—1486
DOI https://doi.org/10.2147/OPTH.S66405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Sohee Jeon, Won Ki Lee
Department of Ophthalmology, Seoul St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Background: We evaluated the efficacy of intravitreal bevacizumab on diabetic macular edema with subfoveal and perifoveal hard exudates.
Materials and methods: Eleven eyes (11 patients) exhibiting diabetic macular edema with subfoveal and perifoveal hard exudates were included in this prospective, nonrandomized interventional pilot study. All patients were treated with monthly scheduled intravitreal bevacizumab injections for 6 months. Changes in the Early Treatment Diabetic Retinopathy Study best corrected visual acuity, amount of hard exudates on fundus photography, and macular edema detected by central subfield thickness on spectral domain optical coherence tomography after six serial injections, were assessed. The amount of hard exudates at each visit was evaluated as pixels in fundus photography, using an Adobe Photoshop program.
Results: Ten of 11 patients completed follow-up. The mean Early Treatment Diabetic Retinopathy Study best corrected visual acuity was 59.9±5.7 letters (Snellen equivalent, 20/63) at baseline evaluation. The best corrected visual acuity exhibited no significant difference at month 6 compared with at baseline (57.9±6.0 letters or 20/70 at month 6; P=0.085). At month 6, mean central subfield thickness decreased from 370.4±56.5 to 334.6±65.0 µm (P=0.009). The mean amount of hard exudates increased from 4467.1±2736.1 to 6592.4±2498.3 pixels at month 6 (P=0.022). No serious adverse events occurred.
Conclusion: Continuous intravitreal bevacizumab was found to have no benefit in visual acuity and amount of hard exudates, despite the improvement of macular edema at 6 months.
Keywords: bevacizumab, diabetic macular edema, hard exudates
Introduction
Diabetic macular edema (DME) is one of the most common causes of visual deterioration in the diabetic population.1 DME has been subdivided into focal and diffuse types.2 Focal macular edema is characterized by focal leakage from microaneurysms and is often associated with hard exudates. The diffuse type of edema is characterized by diffuse leakage from the retinal capillary beds and formation of cystoid spaces. The Early Treatment of Diabetic Retinopathy Study (ETDRS) reported that focal laser treatment to leaking microaneurysms and/or grid treatment to areas of diffuse leakage substantially reduced the risk for visual loss in patients with clinically significant macular edema.3 The ETDRS also revealed that the efficacy of laser treatment was not influenced by the source of leakage (predominantly focal versus intermediate to diffuse), presence of cystoid changes, or severity of hard exudates.4 Since that study, focal/grid laser therapy has been the mainstay of treatment for DME. However, laser treatment only prevents further visual deterioration, rather than improving vision. Furthermore, many clinicians believed that a notable number of patients with diffuse DME remained unresponsive to laser photocoagulation, whereas patients with focal DME responded well to focal laser photocoagulation.5
Vascular endothelial growth factor (VEGF) is overexpressed in diabetic eyes and plays a key role in the development of DME; therefore, anti-VEGF treatment is one of the most promising approaches for the treatment of DME.6,7 Results of several recent prospective studies of DME with ranibizumab consistently revealed that ranibizumab monotherapy or ranibizumab combined with laser treatment were superior to laser treatment alone in terms of functional and anatomical outcomes in center-involving clinically significant macular edema.8–14 Bevacizumab demonstrated similar efficacy in smaller prospective trials.15
Results of these studies were consistent across all subgroups of DME patients, including patients with focal or diffuse DME.13,14 However, patients with DME make up a heterogeneous group of patients with variable clinical manifestations. Many cases of DME have mixed features of focal and diffuse edema, making a clear distinction difficult. Diabetic Retinopathy Clinical Research Network investigators found that the terms “focal DME” and “diffuse DME” were frequently used without clear definitions.16 The evaluation of published information regarding the responses of focal and diffuse DME to treatment and the importance of focal and diffuse DME in assessing prognosis is hindered because the terms are used inconsistently. The investigators suggested that more reproducible methods of grading source and patterns of leakage, area of thickening, and quantitation of hard lipid exudates will need to be devised.16 The best treatment modality or combination of therapeutic modalities for a given patient are yet to be determined.
In this pilot study, we evaluated the efficacy of continuous intravitreal bevacizumab monotherapy in patients exhibiting DME with subfoveal and perifoveal hard exudates at 6 months. In these patients, focal laser treatment was not applicable because some or many of the microaneurysms associated with hard exudates were located within 500 μm of the fovea center.
Methods
This was a prospective, nonrandomized interventional pilot study. The patients were recruited from Retina Service at Seoul St Mary’s hospital between June 16, 2011, and May 30, 2012. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the Seoul Saint Mary’s Hospital Institutional Review Board/Ethics Committee before recruitment. All participants signed an informed consent form after a detailed explanation of the study design, associated investigations for scientific purposes, and adjuvant imaging procedures. The study was registered at www.clinicaltrials.gov under the identifier NCT01422018.
Inclusion criteria were 250 μm or more of central subfield thickness (CST), as measured by spectral domain optical coherence tomography (SD-OCT); fovea-center involving DME; and presence of hard exudates in the perifoveal area, with or without subfoveal involvement. Exclusion criteria were study eyes with any pharmacologic or surgical intervention within 6 months; panretinal or focal/grid laser photocoagulation within 6 months, history of ocular diseases other than diabetic retinopathy, ischemic maculopathy, massive subfoveal hard exudates or subretinal fibrosis, media opacity, pharmacologic intervention on the fellow eye within 3 months, or thromboembolic event within 6 months.
Patients received six consecutive monthly injections of bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA, USA; 1.25 mg in 0.05 mL). A comprehensive ocular examination including ETDRS best corrected visual acuity (BCVA) testing, slit-lamp examination, color fundus photography, macular cube 512×128 scan by SD-OCT, and fluorescein angiography were evaluated as a baseline examination. Follow-up examinations, including ETDRS BCVA and slit-lamp examination, were conducted at every monthly visit, whereas color fundus photography and SD-OCT were conducted at 2-month intervals. BCVA was measured at 4 m with standard ETDRS protocols, using a testing chart transilluminator (Lighthouse International, New York, NY, USA). Visual acuity was scored as the total number of letters read correctly.
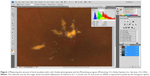
The amount of hard exudates was measured using color fundus photography (Topcon Corporation, Tokyo, Japan) and Photoshop (Photoshop 5.5; Adobe Systems Inc., San Jose, CA, USA), as Smith et al17 previously described, with a few modifications. The fundus images were saved as 24 bit RGB TIFF files, with 256 levels of intensity value for each color channel. The images had minimum resolutions of 2,700 pixels/inch. To achieve a consistent size of each image from a patient, the sizes of the images were adjusted so that the diameter of the optic disc of each image was the same. The amount of hard exudates was determined by the magic wand tool (with adjustment of tolerance of 1 to 5, brush size 5, and zoom to 200%) by the authors. The selected area was measured in pixels (three times for each author), and the average values were taken for the statistical analysis (Figure 1). The amount of hard exudates within 500 μm was also evaluated by the same technique. A concentric circle centered on the fovea with a diameter of 500 μm was made using the built-in caliper tool in the fundus photography instrument, and the amount of hard exudates within the circle was measured.
The SD-OCT was performed with the Cirrus HD-OCT system (software version 3.0, Carl Zeiss Meditec AG, Jena, Germany), according to macular cube 512×128 protocol. The standardized macular cube protocol consisted of 512 horizontal A-scans and 128 vertical B-scan lines with an acquisition time of 2.4 seconds at 6 mm square centered on the fovea. The CST calculated from built-in software was used for statistical analysis.
The serum level of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and glycated hemoglobin level were measured at the initial and final visits.
Statistical analysis was conducted using SPSS (version 12 for Windows; SPSS Inc., Chicago, IL, USA). The Wilcoxon signed-rank test was used to compare the differences between initial and final BCVA, amount of hard exudates, CST, and lipid profiles. Inter- and intraexaminer measuring consistency was determined by having both examiners measure the same image repetitively (ten times each). The bias for each measurement was calculated by the mean difference in data between measurement and examiners; the paired t-test determined whether levels of bias were significantly different from zero, as previously described.18 The limits of agreement, encompassing 95% of differences between two measurements (mean ± [1.96× standard deviation]), were established using the standard deviation of differences.
Results
Eleven eyes were enrolled in this prospective interventional study. The data from ten patients were analyzed because one patient was not present for regular follow-up after the second injection. Baseline characteristics of the enrolled patients are described in Table 1. There were six female and four male patients, with a mean age of 62.9±6.7 years. They were diagnosed with and treated for type 2 diabetes for a mean of 15.3±9.6 years. The stages of diabetic retinopathy were moderate nonproliferative diabetic retinopathy in three eyes, severe nonproliferative diabetic retinopathy in six eyes, and proliferative diabetic retinopathy in one eye. Five patients had been diagnosed with hypertension and were taking oral hypotensive drugs. Nine patients were taking lipid-lowering drugs for hyperlipidemia. At baseline, glycated hemoglobin, triglyceride, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels were 7.1%±0.6% and 245.1±40.6, 208.4±26.1, 49.0±6.6, and 116.8±25.5 mg/dL, respectively (normal ranges are 4.4%–6.0% and 40–200, <200, >60, and <100 mg/dL, respectively). At month 6, no patient was found to exhibit changes in serum lipid and glycated hemoglobin levels more than 20% compared with baseline levels (6.9%±0.5% and 247.4±37.5, 210.6±20.7, 47.6±6.4, and 118.0±25.1 mg/dL, respectively).
Figure 2 presents the treatment outcomes, changes of ETDRS BCVA, CST, and the amount of hard exudates. The mean ETDRS BCVA was 59.9±5.7 letters (Snellen equivalent of 20/63) at baseline and 57.9±6.0 letters (Snellen equivalent of 20/70) at month 6 (P=0.085; Figure 2A). There was no significant difference between the mean values at baseline and each follow-up. Eight patients lost 1–9 ETDRS letters at month 6. One patient was found to have the same ETDRS visual acuity, and another patient gained seven ETDRS letters at month 6.
The mean CST was 370.4±56.5 μm at baseline and improved to 356.6±51.9 μm at month 2 (P=0.041). The CST improved in proportion to repeated injections; as a consequence, CST was 334.6±65.0 μm at month 6 (P=0.009; Figure 2B).
No statistically significant bias was evident with respect to interexaminer and intraexaminer measurements on the amount of hard exudates (Table 2). The mean amount of hard exudates was 4467.1±2736.1 pixels at baseline, but it increased to 6592.4±2498.3 pixels at month 6 (P=0.022; Figure 2C). Only one patient was found to have decreased hard exudates at more than 20% of baseline at month 6; however, five patients were found to have an increase in hard exudates at more than 20% of baseline at month 6.
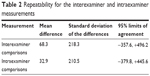  | Table 2 Repeatability for the interexaminer and intraexaminer measurements |
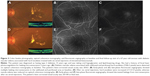
Hard exudates were present within 500 μm from the fovea center in every enrolled eye. The mean amount of hard exudates within 500 μm from the fovea center also exhibited a statistically significant increase, from 322.9±237.5 pixels at baseline to 807.3±536.6 pixels at month 6 (P=0.017). Figure 3 shows the changes in hard exudates of a selected patient. No significant ocular or systemic complications were reported during the follow-up period.
Discussion
In the present study, intravitreal bevacizumab monotherapy had no beneficial effect in DME with subfoveal and perifoveal hard exudates in terms of visual acuity and amount of hard exudates at 6 months of follow-up. Despite a significant reduction of CST, only one of ten eyes showed improved visual acuity after six serial injections. It is notable that visual outcome in this study is different from that of previous studies.8–15 The benefits of ranibizumab were observed as early as 7 days after treatment initiation, signifying a very rapid effect of the drug in DME.14 Over the same time frame of 6 months, the mean gain of visual acuity was 7.24 letters in the ranibizumab group in the Ranibizumab for Edema of the Macula in Diabetes 2 study, despite less-aggressive dosing consisting of four injections.11 In addition, most studies reported favorable visual outcomes regardless of injection schedule; they noted six or more letter improvements in the ranibizumab group at month 6.8–14
Retinal hard exudates consist of lipids and lipoproteins that leak from microaneurysms and dilated capillary segments. Subsequent to laser treatment, hard exudates tend to enlarge and shift centrally with the resolution of macular edema and may decrease after a certain period.20,21 This indicates that precipitation of lipid was induced by the resorption of water and consequent concentration of poorly soluble and poorly diffusible lipids. Our current study shows that the changes in the extent and distribution of hard exudates after continuous intravitreal bevacizumab injections were similar at 6 months. Although the action mechanisms are different, laser and anti-VEGF treatment have a concomitant feature, which has a role in reducing vascular leakage. Anti-VEGF treatment does not have the additional benefit of facilitating resorption of lipid or proteinaceous materials. However, it is possible that reduction of hard exudates and visual improvement might have occurred in our cases at a longer time interval with or without further injections. Nevertheless, our results provide valuable information that has an important clinical implication. Hard exudates are associated with both photoreceptor degeneration and degeneration of neuronal elements in the outer plexiform layer. An increasing amount of hard exudates is independently associated with an increased risk for visual impairment. Rapid resorption of hard exudates as well as intraretinal and subretinal fluid is a desirable endpoint of macular edema treatment.
Several studies have consistently demonstrated that intravitreal triamcinolone resulted in rapid reduction of hard exudates and improvement of visual acuity.22–25 Extravasated lipid materials induce characteristic inflammatory response with involvement of multiple cell types and further accumulation of lipids.26–30 Triamcinolone may have advantages for the resolution of hard exudates by not only stabilizing the blood–retinal barrier31–35 but also exhibiting an anti-inflammatory action that may result in the inhibition of proinflammatory macrophages and leukocytes, prevention of extracellular matrix remodeling,36,37 and inducing differentiation of specific anti-inflammatory macrophages, which have a high capacity for phagocytosis of proinflammatory stimuli such as lipids.37,38 For patients with this type of DME, adding triamcinolone adjunctively to anti-VEGF approach, at least in the initial treatment, may have the benefit of rapid removal of hard exudates.
Elevated serum lipid levels are associated with an increased risk for hard exudates.39 In the present study, nine of ten patients exhibited slightly higher lipid profiles at their initial and final visits, despite lipid-lowering medication and diet control. Tighter lipid control might result in more favorable outcomes regarding the amount of hard exudates in these patients.
The present study showed that after 6 months, there was no beneficial effect of intravitreal bevacizumab for DME with subfoveal and perifoveal hard exudates in terms of visual acuity. Hard exudates increased in extent and tended to move to the foveal side, despite continuous injections. In addition to the short study period, the small number of enrolled patients is another limitation of this study. Further studies with a larger sample size and longer follow-up are required to confirm our study results as well as compare the efficacy of each and combined treatment modalities for this pattern of DME.
Acknowledgments
There was no sponsor or funding organization involved in the study. All authors have completed and submitted the International Committee of Medical Journal Editors Form for Disclosure of Potential Conflicts of Interest. WKL received consultancy honoraria from Novartis and Bayer and lecture fees from Allergan. SJ indicates no financial support or financial conflict of interest. WKL and SJ were involved in the design and conduct of the study, the collection and management of data, and the preparation of the manuscript, and WKL was involved in the review or approval of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. | ||
Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1–32. | ||
The Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report no. 4. Int Ophthalmol Clin. 1987;27(4):265–272. | ||
Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113(9): 1144–1155. | ||
Lövestam-Adrian M, Agardh E. Photocoagulation of diabetic macular oedema − complications and visual outcome. Acta Ophthalmol Scand. 2000;78(6):667–671. | ||
Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. | ||
Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. | ||
Elman MJ, Aiello LP, Beck RW, et al; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. | ||
Elman MJ, Bressler NM, Qin H, et al; Diabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. | ||
Nguyen QD, Shah SM, Heier JS, et al; READ-2 Study Group. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175–2181.e1. | ||
Nguyen QD, Shah SM, Khwaja AA, et al; READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. | ||
Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. | ||
Mitchell P, Bandello F, Schmidt-Erfurth U, et al; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. | ||
Nguyen QD, Brown DM, Marcus DM, et al; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012; 119(4):789–801. | ||
Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130(8):972–979. | ||
Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU; Diabetic Retinopathy Clinical Research Network. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008;146(5):649–655, 655.e1–655.e6. | ||
Smith RT, Nagasaki T, Sparrow JR, Barbazetto I, Klaver CC, Chan JK. A method of drusen measurement based on the geometry of fundus reflectance. Biomed Eng Online. 2003;2(1):10. | ||
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476): 307–310. | ||
Møller F, Bek T. The relation between visual acuity, fixation stability, and the size and location of foveal hard exudates after photocoagulation for diabetic maculopathy: a 1-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2003;241(6):458–462. | ||
Otani T, Kishi S. Tomographic findings of foveal hard exudates in diabetic macular edema. Am J Ophthalmol. 2001;131(1):50–54. | ||
Deák GG, Bolz M, Kriechbaum K, et al; Diabetic Retinopathy Research Group Vienna. Effect of retinal photocoagulation on intraretinal lipid exudates in diabetic macular edema documented by optical coherence tomography. Ophthalmology. 2010;117(4):773–779. | ||
Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol. 2004;88(9):1131–1136. | ||
Avci R, Kaderli B. Intravitreal triamcinolone injection for chronic diabetic macular oedema with severe hard exudates. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):28–35. | ||
Khairallah M, Zeghidi H, Ladjimi A, et al. Primary intravitreal triamcinolone acetonide for diabetic massive macular hard exudates. Retina. 2005;25(7):835–839. | ||
Larsson J, Kifley A, Zhu M, et al. Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol (Copenh). 2009;87(3):275–280. | ||
Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84(9):2995–2998. | ||
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–924. | ||
Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL III. Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110(11):2126–2133. | ||
Nagy L, Szanto A, Szatmari I, Széles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev. 2012;92(2):739–789. | ||
Kruth HS. Macrophage foam cells and atherosclerosis. Front Biosci. 2001;6(1):D429–D455. | ||
Doukas J, Hechtman HB, Shepro D. Endothelial-secreted arachidonic acid metabolites modulate polymorphonuclear leukocyte chemotaxis and diapedesis in vitro. Blood. 1988;71(3):771–779. | ||
Gómez-Ulla F, Marticorena J, Alfaro DV III, Fernández M, Méndez ER, Rothen M. Intravitreal triamcinolone for the treatment of diabetic macular edema. Curr Diabetes Rev. 2006;2(1):99–112. | ||
Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80(4):667–677. | ||
Sears JE, Hoppe G. Triamcinolone acetonide destabilizes VEGF mRNA in Müller cells under continuous cobalt stimulation. Invest Ophthalmol Vis Sci. 2005;46(11):4336–4341. | ||
Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol. 2001;411(3): 231–243. | ||
Hurme M, Siljander P, Anttila H. Regulation of interleukin-1 beta production by glucocorticoids in human monocytes: the mechanism of action depends on the activation signal. Biochem Biophys Res Commun. 1991;180(3):1383–1389. | ||
Waage A, Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988;63(2):299–302. | ||
Ehrchen J, Steinmüller L, Barczyk K, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109(3):1265–1274. | ||
Chew EY, Klein ML, Ferris FL III, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114(9):1079–1084. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.