Back to Journals » Vascular Health and Risk Management » Volume 10
Effect of eprosartan-based antihypertensive therapy on coronary heart disease risk assessed by Framingham methodology in Canadian patients: results of the POWER survey
Authors Petrella R, Tremblay G, De Backer G, Gill D
Received 2 October 2013
Accepted for publication 7 November 2013
Published 29 January 2014 Volume 2014:10 Pages 63—74
DOI https://doi.org/10.2147/VHRM.S55298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Robert J Petrella,1 Guy Tremblay,2 Guy De Backer,3 Dawn P Gill,4,5,6
On behalf of the POWER survey Study Group
1Department of Family Medicine and Cardiology, Lawson Health Research Institute, University of Western Ontario, ON, Canada; 2Centre hospitalier universitaire de Québec, Hôpital du Saint-Sacrement, Sainte-Foy, Québec, QC, Canada; 3Department of Public Health, Ghent University, Ghent, Belgium; 4Aging, Rehabilitation and Geriatric Care Research Centre, Lawson Health Research Institute, London, ON, Canada; 5School of Health Studies, Western University, London, ON, Canada; 6Department of Epidemiology, University of Washington, Seattle, WA, USA
Purpose/introduction: The Canadian Hypertension Education Program (CHEP) has identified blood pressure (BP) control as a key target for an overall reduction in cardiovascular disease risk. The POWER survey (Physicians’ Observational Work on Patient Education According to their Vascular Risk) used Framingham methodology to investigate the impact of an angiotensin-receptor-blocker-based regimen on arterial BP and total coronary heart disease (CHD) risk in a subset of patients recruited in Canada.
Methods: 309 Canadian practices screened for patients with either newly diagnosed or uncontrolled mild/moderate hypertension (sitting systolic blood pressure [SBP] >140 mmHg with diastolic blood pressure [DBP] <110 mmHg). Treatment comprised eprosartan 600 mg/day with add-on antihypertensive therapy after 1 month if required. The primary efficacy variable was change in SBP at 6 months; the secondary variable was the absolute change in the Framingham 10-year CHD risk score.
Results: 1,385 patients were identified, of whom 1,114 were included in the intention-to-treat (ITT) cohort. Thirty-eight point four percent of ITT patients were managed with monotherapy at 6 months, versus 35.2% and 13.7% with two-drug or multiple-drug therapy, respectively. SBP in the ITT cohort declined 22.4 (standard deviation [SD] 14.8) mmHg and DBP declined 10.5 (SD 10.3) mmHg during that time. The absolute mean Framingham score declined 2.1 (SD 3.1) points with significant age and sex variation (P<0.001) and differences between the various Framingham methods used.
Discussion/conclusion: Primary care physicians were able to use a strategy of BP lowering and CHD risk assessment to achieve significant reductions in BP and Framingham-assessed CHD risk. The effect size estimate of the different Framingham methods varied noticeably; reasons for those differences warrant further investigation.
Keywords: blood pressure, hypertension, angiotensin-receptor blocker, observational study
Introduction
Hypertension is widely prevalent among the adult population of Canada and is associated with increased risk of all-cause mortality.1 The expert recommendations of the Canadian Hypertension Education Program2,3 characterize high blood pressure (BP) as “one of the most common modifiable risk factors for cardiovascular disease in Canada” and identify BP control as a key target in any program for the reduction of risk for cardiovascular disease.3
This current Canadian guidance emphasizes the importance of assessing global cardiovascular risk and identifies a range of risk assessment tools that may be used for that purpose, including instruments developed from the Framingham Heart Study.4,5
The POWER project (Physicians’ Observational Work on Patient Education According to their Vascular Risk) created opportunities to evaluate, in patients recruited in Canada, the effect of treatment with the angiotensin-receptor blocker eprosartan on systolic BP (SBP) and, inter alia, the effect of eprosartan-based therapy (EBT) on total coronary heart disease (CHD) risk, as represented by Framingham methodology.
Patients and methods
The overall design and methodology of POWER have been described.6 In brief, POWER was an open-label, post-marketing surveillance survey of 6 months duration. Patients were recruited from Canada and 15 other countries (Bahrain, Belgium, Bulgaria, Croatia, Greece, South Korea, Kuwait, Poland, Qatar, Russia, Saudi Arabia, South Africa, Sweden, Thailand, and the United Arab Emirates). Cardiovascular risk assessment in all countries except Canada was based on the Systemic Coronary Risk Evaluation (SCORE) methodology and is the subject of a separate report.7
Participating physicians collected data for not less than five patients who: 1) had newly diagnosed mild-to-moderate hypertension (defined in the Canada cohort as mean sitting SBP in the range 140 mmHg to <180 mmHg, plus mean sitting diastolic BP [DBP] <110 mmHg) for which eprosartan was proposed as treatment; 2) had hypertension considered not sufficiently controlled by current therapy; or 3) were unable to tolerate other antihypertensive medications. Exclusion criteria were limited to those specified in the extant local Summary of Product Characteristics for eprosartan.8
The protocol stipulated initiation of eprosartan at 600 mg/day. If the BP response after a month of this therapy was considered insufficient, additional drugs (preferably hydrochlorothiazide 12.5 mg/day) could be introduced on a background of continued eprosartan treatment, still at a dose of 600 mg/day.
Participating physicians were encouraged, at their sole discretion, to implement other risk reduction measures as they considered appropriate to the circumstances of individual patients.
Ethical considerations
The design and conduct of POWER in Canada conformed to prevailing requirements relating to conduct of research in humans and the general principles of good clinical practice. Informed written consent was obtained for all patients, who were assured that they were free to withdraw from the study at any time and for any reason without prejudice to their subsequent medical care. Institutional review board and/or ethics committee review and approval was sought and obtained as required by local regulations and practice.
Objectives
The primary objective of POWER was to assess the absolute change in SBP in a large hypertensive population treated with EBT for 6 months.
Secondary efficacy variables included the absolute change in the 10-year risk of hard CHD (myocardial infarction and coronary death) assessed by the Framingham risk scoring from baseline (V1) to final visit (V3).
Three complementary methods were used to calculate the 10-year risk of developing hard CHD in the Canadian contingent:
- The recorded Framingham risk: physicians recorded the Framingham risk estimate using published tables of Framingham point scores.5
- The calculated Framingham risk using tables of the Framingham point scores: risk factors (age, sex, smoking status, SBP, and total and high-density lipoprotein [HDL]-cholesterol) were recorded at each visit and Framingham risk was calculated using exact values of these risk factors.
- Framingham risk was calculated using formulae.
Statistics
BP and laboratory parameters were compared between visits using covariance analysis (with baseline value as the adjusted variable).
Nominal qualitative variables were compared using the chi-squared test. Ordinal qualitative variables were compared using the Wilcoxon test and quantitative variables were compared by the analysis of variance. Descriptive statistics were prepared for safety data on all patients who received at least one dose of EBT.
Results
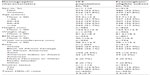
Between May 2005 and October 2009, a total of 1,385 patients were recruited at 309 centers in Canada. The subsequent derivation of a Canadian intention-to-treat (ITT) cohort of 1,114 patients is shown in Figure 1; 933 patients met criteria for a primary prevention population to which the Framingham methodology was applicable. General demographic data for the Canadian ITT cohort and the Framingham-eligible subset appear in Table 1 and baseline hemodynamic information for both those groups appears in Table 2.
The mean duration of treatment in the Canadian ITT population of POWER was 170.6±70.2 days.
Baseline SBP increased with age in the ITT cohort (mean of 157.1±12.9 mmHg at age >70 years versus mean of 153.3±11.3 mmHg at age ≤50 years; P=~0.002 for trend). By contrast, DBP fell with age (84.6±10.4 mmHg at age >70 years versus 94.1±7.3 mmHg at age ≤50 years; P<0.001 for trend). Consequently, pulse pressure (PP) increased considerably with age (72.6±14.0 mmHg at age >70 years versus 59.2±12.3 mmHg at age ≤50 years; P<0.0001).
The proportion of patients with isolated systolic hypertension (SBP ≥140 mmHg and DBP <90 mmHg) in the ITT cohort also increased with age, being 38.2% (n=112) at age 60–69 years and 57% (n=138) at age >70 years, compared with 20.5% (n=50) and 27% (n=90) at <50 years and 50–59 years, respectively (P<0.0001 for trend). Isolated systolic hypertension was in general more prevalent among women than men (41.3% versus 29.5%, respectively; P=0.0003).
Slightly more than half of the Framingham-eligible Canadian contingent of POWER (n=510; 54.7%) was initially assigned to monotherapy to control BP, 24% (n=226) were assigned to two-drug therapy, and 10.4% (n=97) to multidrug therapy. Data were not recorded for 10.7% of patients (n=100). During the survey, that distribution shifted away from monotherapy (38.4% [n=358] at completion of follow-up) toward greater use of bitherapy (35.2% [n=328] at completion of follow-up) or multi-drug regimens (13.7% [n=119] at completion of follow-up). Combination therapy was more often encountered in older or diabetic patients, and in those with significant cardiovascular history. The most often recorded drugs supplementing eprosartan at V3 were: hydrochlorothiazide in a fixed dose combination (n=337; 38%); calcium channel blockers (n=96; 10.8%); ACE (angiotensin-converting-enzyme)-inhibitors (n=63; 7.1%); and beta-blockers (n=49; 5.5%).
Some 37% of the Framingham-eligible patients (n=349) had a family history of cardiovascular disease. Also recorded in that contingent were diabetes (n=129), microalbuminuria (n=58), arteriosclerosis (n=35), left ventricular hypertrophy (n=29), raised plasma creatinine (n=29), and proteinuria (n=20).
Baseline Framingham risk status by each of the three methods used is illustrated in Figure 2. The mean value calculated by formulae was 7.8%±6.3% with evidence of a marked sex difference (men 11.1%±6.9%, women 3.9%±1.7%; P<0.001). There was also a marked progression of risk score with age (4.0%±2.8% at age <50 years, 6.9%±3.7% at 50–59 years, 9.0%±6.0% at 60–69 years, and 11.2%±9.5% at age ≥70 years). The range of risk encountered precluded estimation of a mean value by the calculation method.
  | Figure 2 Baseline Framingham CHD risk status, according to methodology. |
BP trends during treatment
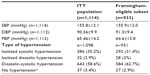
Among the overall ITT cohort, mean SBP declined 22.4±14.8 mmHg to 133.5±13.6 mmHg during the course of the survey. Mean DBP declined 10.5±10.3 mmHg to 79.8±8.2 mmHg and mean PP fell 11.82±14.4 mmHg to 53.8±13.3 mmHg. The mean reduction in PP was greater in women than in men (12.9±15.1 mmHg for women versus 11.0±13.6 mmHg for men; P=~0.03), even though there were no significant differences in mean changes in SBP or DBP.
The mean reduction in both SBP and DBP was ~3 mmHg larger in younger patients (up to age 59 years) than in older ones (≥60 years; P<0.0088 for SBP trend, P<0.001 for DBP trend). However, there was no significant age-related variation in the mean PP reduction.
Patients with no history of cardiovascular disease at baseline (ie, those eligible for calculation of risk by Framingham methodology) had ~3–4 mmHg greater mean reduction than history-positive patients in SBP (22.9 mmHg versus 19.2 mmHg, respectively) and DBP (11 mmHg versus 7 mmHg, respectively) (both P<0.0001). The reduction in mean PP was also larger in the Framingham-eligible cohort than in the ITT population, but the difference (~1.5 mmHg), although significant (P<0.0001), was small.
In all, 885 patients (94.9%) of the Framingham-eligible contingent of 933 patients were considered to have displayed a response to BP-lowering therapy by the final visit of the study, according to the definition of “response” as either: SBP <140 mmHg and/or a reduction of SBP of ≥15 mmHg; or DBP <90 mmHg and/or reduction of DBP of ≥10 mmHg. In parallel, 659 patients (70.6%) were classified as having normalized BP at V3 (defined as SBP <140 mmHg and DBP <90 mmHg).
Trends in other CHD risk factors
The number of smokers in the Framingham-eligible subset fell from 153 at baseline (16.4% of 933) to 128 at V3 (15.1% of 846). Baseline mean total cholesterol was 204.5±38.0 mg/dL, which declined to 197.2±37.4 mg/dL at V3, a mean reduction of 9.1±29.2 mg/dL. Similar small reductions were recorded in the overall Canadian ITT population.
Risk score trends during treatment
Figures 3 and 4 depict the shifts in distribution of the Framingham CHD risk distribution by the end of the survey according to the various methods used.
  | Figure 4 Aggregate shift in Framingham CHD risk distribution during treatment. |
The absolute mean score (determined by formulae) declined by 2.1±3.1 points (n=374), with evidence of variation according to age and sex (Table 3).
Shifts in BP, cholesterol (total and HDL), and smoker status for patients who were recorded as achieving a reduction in Framingham-estimated CHD risk of at least one category during 6 months of EBT, are shown in Table 4. Improvements in the non-BP variables were more likely to be noted when Framingham scores were derived from physician recordings.
Safety findings
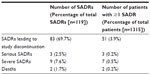
A total of 119 suspected adverse drug reactions were recorded in 76 patients (5.8%) in the safety population (n=1,315). Summary details of these incidents are provided in Table 5.
The two deaths (which also accounted for two of the serious events recorded) were fatal myocardial infarctions in male patients aged 57 and 67, respectively. The third serious event was recorded as a case of ileal inflammation that resolved and did not require termination of that patient’s participation in the study.
The severe events (nine events in seven patients) were recorded across a wide range of organ-systems and manifestations and produced no coherent safety signal. In detail, these events were headache (two events in two patients), nausea (one event in one patient), gouty arthritis (one event in one patient), dizziness (one event in one patient), cough (one event in one patient), and sense of suffocation (one event in one patient). The remaining two severe events (in one patient) were not coded to an organ-system.
Among suspected adverse drug reactions leading to termination of participation in the study, the largest single contributing organ-system was ‘nervous system disorders’, of which headache (15 cases in 15 patients) and dizziness (eleven cases in eleven patients) were the biggest categories by a substantial margin. Fatigue (six cases in six patients) and cough (four cases in four patients) were other categories contributing more than two cases.
Discussion
The significance of primary care as a route to improved cardiovascular health for Canadians is acknowledged. Optimizing the performance of primary care for that purpose is a multifactorial challenge, involving attention to organizational structures and incentive mechanisms as well as core medical competencies.9,10 Convincing doctors that they have effective, reliable, and easy-to-use tools for the evaluation and management of cardiovascular risk is nevertheless central to the delivery of sound primary care. Our experience in the Canadian contingent of POWER indicates that risk assessment and monitoring based on the Framingham instruments is feasible and effective, though that conclusion is subject to various qualifications.
We used three methods to calculate cardiovascular risk. As shown in Figure 2, there was reasonably close correspondence between the baseline risk distribution provided by the two calculated methods. There was also fair correspondence between these methods in the shift in risk distribution during EBT (Figure 3). By contrast, the risk distribution obtained from physicians’ estimates produced a notably higher proportion of high-risk (≥15%) patients than the other methods (Figure 2) and a different pattern of risk redistribution during treatment (Figure 3). Further inspection of the data in Figure 3 reveals that, although physician estimates conformed with the other two methods in registering a shift of patients from higher to intermediate levels of risk, they were less sensitive to movements from intermediate to low risk levels. The overall consequence of this discrepancy is evident in Figure 4, which shows that the percentage of patients judged to have improved their Framingham risk status during the period of treatment and observation was 28% by physician estimates but more than 40% by the two other methods.
The discrepant estimates produced by these different applications of the Framingham risk scoring method were not anticipated when the study was being devised. We do not know if this is an isolated finding attributable to chance; nor do we know which of the two estimates (28% versus >40%) is a truer reflection of the real effect of treatment, though the congruence between the calculation methods leads us to favor those findings. Individual physicians were not advised of the differences between methods during the trial and therefore would not have been aware of the anomaly. Even without comparisons, however, it is possible that some physicians would have been discouraged to find (from their own estimates) that their efforts appeared to have demonstrably improved the risk status of only about one patient in four. POWER was not designed to investigate how physicians’ assessment of the effectiveness of an intervention may affect their motivation to persist with that intervention, but our observations lead us to think that this is an area of primary care practice that deserves more attention. Initiatives such as Canadian Health Awareness program may prove informative in this respect.11
The scale of the treatment effect observed may also have been influenced by the fact that we used the Framingham assessment for 10-year risk of hard CHD. Experience elsewhere in North America12 confirms that, as might be expected, application of the Framingham global cardiovascular instrument, which adds cerebrovascular disease, angina, heart failure, and peripheral vascular disease to the endpoints of the CHD instrument, displaces the distribution toward higher levels of risk. Such a redistribution might increase the potential for demonstrating an effect of intervention, especially when the effects of sartans on stroke are considered.13–16 In any event, it should be borne in mind that change in a score does not necessarily signify a change in actual risk.
Although POWER was configured to examine overall risk, the principal intervention was against BP, and it was reduction in BP that drove the realignment of overall CHD risk (Table 4). By the current standards of North America, ours was a cohort with relatively low total cholesterol and rates of smoking.17 It may be conjectured that similar overall effect on risk scores might be achieved by other interventions, with similar effect on BP-related risk. However, it may be noted that Framingham Heart Study researchers have recently suggested that impaired vascular function may contribute to increase in cardiovascular risk by promoting exaggerated BP responses during the physical activities of daily living.18 Reports that angiotensin-receptor blockers can ameliorate both these aspects of vascular dysfunction19–22 may therefore be supportive of a degree of treatment-specific benefit (although, see Sozen et al23 for reservations that any effect on endothelial function may not be sustained in long-term clinical use).
Detailed examination of outcomes by ethnic identity was not part of our analysis plan and, in any event, would necessarily have been restricted to the black or white Canadians who comprised three-quarters of our cohort. Appreciation of the variations in exposure to cardiovascular risk factors among Canadians of differing ethnicities is important,24 though we incline also to the view that duration of residency and degree of acculturation are likely to be substantial influences on this aspect of cardiovascular risk.25
In conclusion we were able to demonstrate, in this cohort of Canadian patients with hypertension, that 6 months of EBT was associated with substantial reductions in total CHD risk, and that Framingham instruments could be used successfully in primary care for the initial assessment of risk and to monitor the effect of therapy. The effect size observed varied noticeably according to the Framingham method used, for reasons that remain unclear but which may warrant further investigation.
Acknowledgments
The authors wish to thank the physicians and patients who participated in the POWER survey: Ibrahim Abdul-Rahman, Oshawa, ON, Canada; Taiwo Aderibigbe, Hamilton, ON, Canada; Ronald Akhras, Montreal, QC, Canada; Sanjiv Anand, Dieppe, NB, Canada; Charles Clifford Anderson, Upper Tantallon, NS, Canada; Wameed Ateyah Schomberg, ON, Canada; Sylvia Athaide, North York, ON, Canada; Murray Awde, London, ON, Canada; Steve Bacskai, Ottawa, ON, Canada; Abdoulaye Bah, Montreal, QC, Canada; Robert Baker, Kamloops, BC, Canada; Teodor P Barbarosie, Mont-Laurier, QC, Canada; Ginette Barriere, Sainte Catherine, QC, Canada; Gary Bryan Barrs, Verdun, QC, Canada; Denis E Beaulieu, Trois-Pistoles, QC, Canada; Denis Beaumont, Mont-Joli, QC, Canada; Nicholas Bedard, Gatineau, QC, Canada; Pierre Bégin, Laval, QC, Canada; Richard Bernard, Trois-Rivières, QC, Canada; Trevor Berns, Aurora, ON, Canada; Alfi Beshay, St Catharines, ON, Canada; Gunvant Bhatt, Prescott, ON, Canada; Eric Bicrell, Saint-Léonard, QC, Canada; Serge Bilodeau, Jonquière, QC, Canada; Gary Bloomberg, Burnaby, BC, Canada; Guy Boucher, Rimouski, QC, Canada; J P Michel Bouffard, Vanier, ON, Canada; Angelo-Edouard Bourkas, Montreal, QC, Canada; William Bowler, London, ON, Canada; Jeannot Breton, Plessissville, QC, Canada; Yvon Bricault, Laval, QC, Canada; Thomas Brien, CBS, NL, Canada; Gilles Brouillette, Lasalle, QC, Canada; Julie Cabot, Thetford Mines, QC, Canada; Mathieu Caron, Laval, QC, Canada; David Carswell, Harrow, ON, Canada; Victor Carulei, Assiniboia, SK, Canada; James Casserly, Ottawa, ON, Canada; Benoit Castonguay, Rimouski, QC, Canada; Edouard Cattan, Orleans, ON, Canada; Serge Champoux, Jonquière, QC, Canada; Jacques Charbonneau, Sainte-Julie, QC, Canada; Arif Rahman Chaudhri, Weston, ON, Canada; Claudius Che, Fort Erie, ON, Canada; Raja Chehayeb, Greenfield Park, QC, Canada; Kanchana Chiamvimonvat, Orleans, ON, Canada; Chung Fok Chiu, North York, ON, Canada; Tomas Cieza, Chicoutimi, QC, Canada; Terrence Clarke, Gatineau, QC, Canada; Louise Comeau, Montreal, QC, Canada; Pierre Comtois, Montreal, QC, Canada; Johannes Conradie, Kelowna, BC, Canada; James Conway, Smith Falls, ON, Canada; Paul Coolican, Morrisburg, ON, Canada; Juan L Cordova, Winnipeg, MB, Canada; Michel C Côté, Saint-Henri-de-Lévis, QC, Canada; Charles Courchesne, Boucherville, QC, Canada; Aurelian Cristache, Montreal, QC, Canada; Michael Csanadi, Fort Erie, ON, Canada; Greg Curnew, Hamilton, ON, Canada; Anthony G Czaharyn, Kirkland, QC, Canada; Olayinka Dada, Hamilton, ON, Canada; Michel Dagenais, Sainte-Julie, QC, Canada; Jean-Marc Daigle, Cowansville, QC, Canada; Michel Daneault, Ste-Julienne, QC, Canada; Angela Ngoc Dung Dang, Montreal, QC, Canada; Luc Dauphinais, St-Lin Laurentides, QC, Canada; Faroux Dawood, Waterloo, ON, Canada; Selwyn De Souza, Ottawa, ON, Canada; Jagdish Desai, Brantford, ON, Canada; Nicole Desmarais, Hanmer, ON, Canada; Deepinderjit Dhatt, Sudbury, ON, Canada; Nav Dhiraj, Milton, ON, Canada; Kien Quan Diec, Anjou, QC, Canada; Jean-François Dionne, Sherbrooke, QC, Canada; Wayne Domanko, Morrisburg, ON, Canada; Anthony Dowell, Pointe Claire, QC, Canada; William Doyle, Sarnia, ON, Canada; André Ducharme, St-Lin Laurentides, QC, Canada; Michelle Dumais, L’ Anse St-Jean, QC, Canada; Guy Dumas, St-Léonard, QC, Canada; John Dundon, Kitchener, ON, Canada; Hong-Huy Duong, Montreal, QC, Canada; William Enright, Windsor, NS, Canada; Fakhruddin Essaji, Mt. Pearl, NL, Canada; Kirby Evans, New Market, ON, Canada; Dan Ezekiel, Vancouver, BC, Canada; Anne Faber, Loretteville, QC, Canada; Mohamed Faizer, Cornwall, ON, Canada; Jairie Ferasol, Windsor, ON, Canada; Louis Fields, Thornhill, ON, Canada; Victor John Figurado, Mississauga, ON, Canada; Pierre Filteau, St-Marc-des-Carrieres, QC, Canada; Philip Fingrut, North York, ON, Canada; Gérald Fortier, Jonquière, QC, Canada; Christian Fortin, Jonqière, QC, Canada; Pierre Fournier, Plessissville, QC, Canada; Le Roy Franklin, Mississauga, ON, Canada; Lucien Paul Fruchtermann, Montréal, QC, Canada; Joachim Fuchs, Duncan, BC, Canada; Gary Fullerton, Woodstock, ON, Canada; Carl-Eric Gagné, Trois-Rivières, QC, Canada; Robert Gagnon, Clermont, QC, Canada; Gilles Gaudreau, Sorel-Tracy, QC, Canada; Andre Gauthier, Vanier, ON, Canada; Michel Gauthier, Azilda, ON, Canada; J David Giddens, Toronto, ON, Canada; Hamid Jamal Gilani, Guelph, ON, Canada; Sylvie Gill, Sorel-Tracy, QC, Canada; Luc Girard, Bécancour, QC, Canada; Bronte Golda, Hamilton, ON, Canada; Martin Goldstein, Kirkland, QC, Canada; Mark Goodbaum, Thornhill, ON, Canada; Francois Gougoux, Cacouna, QC, Canada; Pierre Goyer, Laval, QC, Canada; Ronald Goyer, St-Eustache, QC, Canada; Steve Graham, Montreal, QC, Canada; Serge Gravel, Trois-Pistoles, QC, Canada; Louis Grenier, Saint-Agapit, QC, Canada; Jeanne Guillemette, Sainte-Foy, QC, Canada; Taras Gwozdecki, Winnipeg, MB, Canada; Jeffery Habert, Thornhill, ON, Canada; Samir Habib, Montreal, QC, Canada; Nader Habib, Laval, QC, Canada; Antoine Hani, Montreal, QC, Canada; Normand Harvey, La Malbaie, QC, Canada; Youssef Hassan, Trois-Rivières, QC, Canada; Banafcheh Hejazi, Montreal, QC, Canada; Simon Hevey, Roberval, QC, Canada; P J Hierlihy, Ottawa, ON, Canada; James Hii, Vancouver, BC, Canada; Michael Ho, Toronto, ON, Canada; Ngoc-Vinh Hoang, Montreal, QC, Canada; Tommy Hong, Mississauga, ON, Canada; Gabriel Houle, St-Fabien, QC, Canada; Chantal Huppé, Gatineau, QC, Canada; Gary Ing, Windsor, ON, Canada; Ivan Jagas, Kitchener, ON, Canada; Ajay Jagota, Pickering, ON, Canada; Romulo Javier, Mississauga, ON, Canada; Pierre Juery, Ottawa, ON, Canada; Alan Kassel, North York, ON, Canada; Anu Kaushal, Richmond Hill, ON, Canada; John Kelly, Victoria, BC, Canada; Andrew Kiellerman, Victoria, BC, Canada; James Kim, Brampton, ON, Canada; Matthew C Young Kim, Toronto, ON, Canada; Jacobus Kooy, Penticton, BC, Canada; Allan Kopyto, Hamilton, ON, Canada; Evangelous Kouros, Welland, ON, Canada; Michoke Krisdaphongs, Conception Bay South, NL, Canada; Andrew Kuchtaruk, Sudbury, ON, Canada; Pierre Kugler, Waterloo, ON, Canada; Arthur Kushner, Etobicoke, ON, Canada; Gilles Labbé, Lévis, QC, Canada; Roger Laberge, Chateauguay, QC, Canada; Roger Lahens, Longueuil, QC, Canada; Alain-Paul Lalonde, St-Bruno, QC, Canada; Andy Lam, Grimsby, ON, Canada; Guylaine Landry-Frechette, St-Wenceslas, QC, Canada; Gérald Lapointe, Boucherville, QC, Canada; Claude Laroche, Montreal, QC, Canada; Gilles Laurin, Saint-Jérôme, QC, Canada; Pierre Michel Laurin, St-Jérôme, QC, Canada; Christian Lauriston, Montreal, QC, Canada; Denis Lavigueur, Montréal, QC, Canada; Normand Lavoie, Rosemère, QC, Canada; Shelagh Leahey, Yarmouth, NS, Canada; Ma Christina Ledesma-Cadhit, Toronto, ON, Canada; Jean-Jacques Légaré, St-Eustache, QC, Canada; Ceta Leung, Scarborough, ON, Canada; Alex Leung, Kamloops, BC, Canada; Robin Lévesque, Ste-Anne- des-Plaines, QC, Canada; Louis Charles Levros, Montreal, QC, Canada; Wally Liang, Windsor, ON, Canada; Richard Longtin, La Prairie, QC, Canada; Benoit Loranger, St-Eustache, QC, Canada; Richard Lortie, Longueuil, QC, Canada; Talaat Lotfallah, Kingston, ON, Canada; Tse Luk, Winnipeg, MB, Canada; Robert Luton, London, ON, Canada; Gary Magee, Thornhill, ON, Canada; Ranjith Mahadeva, Stirling, ON, Canada; Michael Malek, Ottawa, ON, Canada; Gérard Maltais, St-Bruno, QC, Canada; Elias Maraghi, Hastings, ON, Canada; Pierre Martin, Trois-Rivières, QC, Canada; Mario Martineau, Lavaltrie, QC, Canada; Ricardo Martinez, Saint-Laurent, QC, Canada; Valdemar Martinho, Ottawa, ON, Canada; Yvan Mathieu, Sainte-Marie, QC, Canada; Thomas McGarry, Trepassy, NL, Canada; Joseph McIsaac, Ottawa, ON, Canada; Keith McQueen, Victoria, BC, Canada; Xavier Medina, Scarborough, ON, Canada; Upender Mehan, Cambridge, ON, Canada; Aaron M Mellon, Winnipeg, MB, Canada; Gloria S Meneses, Scarborough, ON, Canada; Hamid Mesbahi, Montreal, QC, Canada; Jean Claude Michaud, Rivière-du-Loup, QC, Canada; Adina Moldoveanu, Scarborough, ON, Canada; Karen Moran de Muller, Winnipeg, MB, Canada; Michel Morissette, Saint-Agapit, QC, Canada; Pierre Morissette, Paspébiac, QC, Canada; Marie-Hélène Morval, Ste-Julie, QC, Canada; William Moulton, Marystown, NL, Canada; Adi Mudaliar, Vancouver, BC, Canada; Charles Myers, Victoria, BC, Canada; Shantha Nada-Rajah, Mississauga, ON, Canada; Houshang Naimi, Beaconsfield, QC, Canada; Daniel Neault, Alma, QC, Canada; Simon Ngui, Vancouver, BC, Canada; Dinh Hao Nguyen, Montreal, QC, Canada; Paul Nijmeh, Scarborough, ON, Canada; As Nirwan, Victoria, BC, Canada; Lionel Noronha, Stirling, ON, Canada; Osbourne Isaac Noronha, Belleville, ON, Canada; Ien Oei, Sydney, NS, Canada; Shekhar Pandey, Cambridge, ON, Canada; Patrick Pang, Niagara Falls, ON, Canada; John Paolone, St Catharines, ON, Canada; Serge Paquette, Laval, QC, Canada; Gelasio Parlan JR, Mississauga, ON, Canada; Prafulchandra Patel, Winnipeg, MB, Canada; Eric Pauyo, Lasalle, QC, Canada; Mario Payette, Saint-Bruno, QC, Canada; Jonathan Peet, Waterloo, ON, Canada; Marcel Pelland, Sorel-Tracy, QC, Canada; Marie-France Pelletier, Mercier, QC, Canada; Michael Perley, Woodstock, NB, Canada; Guy Perrier, La Sarre, QC, Canada; Robert Petrella, London, ON, Canada; Howard Petroff, Pickering, ON, Canada; Paul Phelan, Kensington, PE, Canada; Wayne Phillipson, North Bay, ON, Canada; Michel Pineau, Trois-Pistoles, QC, Canada; Jacques Piuze, Thetford Mines, QC, Canada; Ludovic Plante, Sherbrooke, QC, Canada; Francisco Portugal, Toronto, ON, Canada; Ralph Profetto, Stoney Creek, ON, Canada; Robert Prosser, Oromocto, NB, Canada; Jean Proulx, Joliette, QC, Canada; Brian Ramjattan, St. John’s, NL, Canada; Koshela Ranjith, Stirling, ON, Canada; Yves Raymond, Rivière- du-Loup, QC, Canada; Yasmin Rehemtula, Mississauga, ON, Canada; Pierre Rhéaume, Boucherville, QC, Canada; Cyril Riche, Mt Pearl, NL, Canada; Claude Roberge, St-Stanislas, QC, Canada; Bernard Roberts, Marystown, NL, Canada; Julie Rodrigue, Orleans, ON, Canada; Daniel Rooyakkers, Seaforth, ON, Canada; Bruno Roy, Beauceville, QC, Canada; Carolyne Roy, Vanier, ON, Canada; Richard Ruest, Montreal, QC, Canada; Howard Rufdner, Toronto, ON, Canada; Linda Sabetti, Mississauga, ON, Canada; Tariq Saeed, Mississauga, ON, Canada; Wade Sahheed, Mississauga, ON, Canada; Colin Saldanha, Mississauga, ON, Canada; Dioscoro Sarile, Toronto, ON, Canada; Earl Schwartz, Toronto, ON, Canada; James Seaman, Kentville, NS, Canada; Samuel Serfaty, Montreal, QC, Canada; Shivani Sharma, Markham, ON, Canada; Chung Shih, Woodbridge, ON, Canada; Ian Shiozaki, Newboro, ON, Canada; John Shubat, Lucknow, ON, Canada; Stewart James Silagy, Winnipeg, MB, Canada; Jonathan Singerman, Montreal, QC, Canada; Gurdayal Singh, Vancouver, BC, Canada; Jayaditya Sinha, Pickering, ON, Canada; Edward Smith, Vanier, ON, Canada; Vincente Somera, Lasalle, QC, Canada; Mohunlall Soowamber, Brossard, QC, Canada; Robert Stevenson, Sussex, NB, Canada; Salim Sunderji, London, ON, Canada; Joseph Sylvain-Lucien, Grand Mère, QC, Canada; Lucas Tai, Toronto, ON, Canada; Nathalie Talbot, Daveluyville, QC, Canada; John Taliano, St Catherines, ON, Canada; Pooi-Lin Tham, London, ON, Canada; Francois Theoret, Hawkesbury, ON, Canada; Daniel R Toledano, Toronto, ON, Canada; Hong Phuc Tran-le, Val-d’Or, QC, Canada; Serge Tremblay, Forestville, QC, Canada; Dimitri-Emmanuel Treymann, Montreal, QC, Bich Hang Trinh, Kirkland, QC, Canada; Nelson Ubani, Montreal, QC, Canada; Stan G Van Duyse, Montreal, QC, Canada; Marie-Claude Vandal, Quebec, QC, Canada; Astghik Vartanian, Montreal, QC, Canada; Michael Vecchio, North York, ON, Canada; Christian Vinette, Daveluyville, QC, Canada; Paul Walsh, Holyrood, NL, Canada; John Wilcynzki, Niagara Falls, ON, Canada; Hany William, Cornwall, ON, Canada; Clem Williams, West Vancouver, BC, Canada; Ewa Wojtowska, Vancouver, BC, Canada; Danny HK Wong, Richmond, BC, Canada; Adrian Woodrow, North York, ON, Canada; Henry Wrobel, Regina, SK, Canada; Michael Wyman, North York, ON, Canada; Mayer Yacowar, Innisfil, ON, Canada; David Yanover, Hamilton, ON, Canada; Daniel Yim, North York, ON, Canada; Thomas Zaphiratos, Montreal, QC, Canada; Louis Stephen Zavodni, Hamilton, ON, Canada. Preparation of this report was assisted by Hughes associates, Oxford, UK.
Disclosure
RJP reports research grants from Pfizer, Astra-Zeneca, Novartis, Abbott, and Sanofi-Aventis, and honoraria from Abbott, Novartis, and Sanofi-Aventis for speaking engagements and symposia participation. GT reports speaker fees from Abbott, Astra-Zeneca, Servier, and Merck Sharp and Dohme. GDB reports research grants from Merck Sharp and Dohme and speaker fees from Astra-Zeneca. DG reports no conflicts of interest in this work. The POWER study is supported financially by Abbott Products Operations AG, Hegenheimermattweg 127, 4123 Allschwil, Switzerland.
References
Robitaille C, Dai S, Waters C, et al. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ. 2012;184(1):E49–E56. | |
Daskalopoulou SS, Khan NA, Quinn RR, et al; Canadian Hypertension Education Program. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270–287. | |
Padwal RS, Hemmelgarn BR, Khan NA, et al; Canadian Hypertension Education Program. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 1 – blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2009;25:279–286. | |
Grover SA, Hemmelgarn B, Joseph L, Milot A, Tremblay G. The role of global risk assessment in hypertension therapy. Can J Cardiol. 2006;22:606–613. | |
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. | |
De Backer G, Petrella RJ, Goudev AR, Radaideh GA, Rynkiewicz A, Pathak A. Design and methodology of POWER, an open-label observation of the effect of primary care interventions on total cardiovascular risk in patients with hypertension. Fundam Clin Pharmacol. 2013;27:210–215. | |
De Backer G, Petrella RJ, Goudev AR, Radaideh GA, Rynkiewicz A, Pathak A. Effect of antihypertensive therapy on SCORE-estimated total cardiovascular risk: results from an open-label, multinational investigation -the POWER survey. Int J Hypertens. Epub July 25, 2013. | |
Health Canada [database on Internet]. Available from: http://webprod5.hc-sc.gc.ca/dpd-bdpp/info.do?code=67885&lang=eng. Accessed December 11, 2013. | |
Liddy C, Hogg W, Russell G, et al. Improved delivery of cardiovascular care (IDOCC) through outreach facilitation: study protocol and implementation details of a cluster randomized controlled trial in primary care. Implement Sci. 2011;6:110. | |
Campbell N, Young ER, Drouin D, et al. A framework for discussion on how to improve prevention, management, and control of hypertension in Canada. Can J Cardiol. 2012;28:262–269. | |
Carter M, Karwalajtys T, Chambers L, et al; CHAP Working Group. Implementing a standardized community-based cardiovascular risk assessment program in 20 Ontario communities. Health Promot Int. 2009;24:325–333. | |
Tattersall MC, Karmali KN, Gangnon RE, Keevil JG. The population effects of the global cardiovascular risk model in United States adults: findings from the National Health and Nutrition Surveys, 2005–2006. J Clin Lipidol. 2011;5:166–172. | |
Lu GC, Cheng JW, Zhu KM, Ma XJ, Shen FM, Su DF. A systematic review of angiotensin receptor blockers in preventing stroke. Stroke. 2009;40:3876–3878. | |
Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. | |
Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. | |
Schrader J, Lüders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36:1218–1226. | |
Steiner S, Helis E, Chen L, et al. A cross-national comparative study of blood pressure levels and hypertension prevalence in Canada and Hungary. J Hypertens. 2012;30:2105–2111. | |
Thanassoulis G, Lyass A, Benjamin EJ, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843. | |
Andreadis EA, Sfakianakis ME, Tsourous GI, et al. Differential impact of angiotensin receptor blockers and calcium channel blockers on arterial stiffness. Int Angiol. 2010;29:266–272. | |
Inaba S, Iwai M, Furuno M, et al. Temporary treatment with AT1 receptor blocker, valsartan, from early stage of hypertension prevented vascular remodeling. Am J Hypertens. 2011;24:550–556. | |
Ono Y, Nakaya Y, Bando S, Soeki T, Ito S, Sata M. Telmisartan decreases plasma levels of asymmetrical dimethyl-L-arginine and improves lipid and glucose metabolism and vascular function. Int Heart J. 2009;50:73–83. | |
Iino K, Watanabe H, Iino T, et al. Candesartan improves impaired endothelial function in the human coronary artery. Coron Artery Dis. 2012;23:278–283. | |
Sozen AB, Kayacan MS, Tansel T, et al. Drugs with blocking effects on the renin-angiotensin-aldosterone system do not improve endothelial dysfunction long-term in hypertensive patients. J Int Med Res. 2009;37:996–1002. | |
Liu R, So L, Mohan S, Khan N, King K, Quan H. Cardiovascular risk factors in ethnic populations within Canada: results from national cross-sectional surveys. Open Med. 2010;4:e143–e153. | |
Chiu M, Austin PC, Manuel DG, Tu JV. Cardiovascular risk factor profiles of recent immigrants vs long-term residents of Ontario: a multi-ethnic study. Can J Cardiol. 2012;28:20–26. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.