Back to Journals » Local and Regional Anesthesia » Volume 12
Effect of bupivacaine and adjuvant drugs on skeletal muscle tissue oximetry and blood flow: an experimental study
Authors Schubert AK, Müller S, Wulf H , Steinfeldt T , Wiesmann T
Received 30 January 2019
Accepted for publication 2 July 2019
Published 29 August 2019 Volume 2019:12 Pages 71—80
DOI https://doi.org/10.2147/LRA.S203569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Ann-Kristin Schubert,1 Stefan Müller,1 Hinnerk Wulf,1 Thorsten Steinfeldt,1,2 Thomas Wiesmann1
1Department of Anesthesiology and Intensive Care Medicine, University Hospital Marburg, Philipps University of Marburg, Marburg, Germany; 2Department of Anesthesiology and Intensive Care Medicine, Diakoniekrankenhaus Schwäbisch Hall, Schwäbisch Hall, Germany
Correspondence: Thomas Wiesmann
Department of Anesthesiology and Intensive Care Medicine, University Hospital Marburg, Philipps University of Marburg, Baldingerstraße, Marburg 35033, Germany
Tel +49 6 421 586 9362
Email [email protected]
Background: Skeletal muscle microvascular blood flow plays a critical role in many myopathologies. The influence of bupivacaine and adjuvants on skeletal muscle microvascular perfusion and tissue oximetry is poorly understood but might be a relevant risk factor for myopathies after local anesthetic administration. The aim of this experimental study was to determine the effects of bupivacaine alone or in combination with epinephrine or clonidine on skeletal muscle perfusion and tissue oximetry.
Methods: Combined tissue spectrophotometry and Laser-Doppler flowmetry and tissue oximetry were used to assess local muscle blood flow in anesthetized pigs after topical administration of test solutions (bupivacaine, bupivacaine with epinephrine or clonidine, saline). Measurements were performed for up to 60 mins.
Results: The application of bupivacaine alone did not alter relative muscle blood flow significantly, whereas the addition of epinephrine or clonidine to bupivacaine resulted in a significant reduction of relative muscle blood flow at T30 and T60. However, bupivacaine resulted in a significant decrease of tissue oximetry values when compared to saline control group at T30 and T60. The application of bupivacaine combined with clonidine or epinephrine resulted in no significant reduction of tissue oximetry when compared to bupivacaine alone.
Conclusion: Bupivacaine alone results in a significant decrease of tissue oximetry in skeletal muscle which is not increased by the addition of epinephrine or clonidine despite further reductions of microcirculatory perfusion. Overall, bupivacaine alone or with adjuvants does produce local muscle ischemia for which pathological consequences need to be addressed in further studies.
Keywords: bupivacaine, adjuvants, tissue perfusion, tissue oxygenation, ischemia
Introduction
Local anesthetics (LAs) reversibly inhibit specific Na+ channels in neurons and prevent propagation of action potential along peripheral nerves. In regional anesthesia, LAs are injected in proximity to the target nerve in the surrounding tissue. In most peripheral nerve blockades, the surrounding tissue consists of skeletal muscle. After injection, the LA molecules spread and diffuse through the tissue and finally reach their intracellular target structure within neural tissue. A larger proportion of the LA spreads through the surrounding tissue and exhibits myotoxic and vasoactive effects.1–5 The consequences of this inadvertent spread are known as myotoxic effects of LAs on skeletal muscle which are well described especially for bupivacaine1,3,6 by increased intracellular calcium levels and a breakdown of intracellular homeostasis7 as well as inhibition of mitochondrial function.8 Interestingly, the long-known vasoactive effects of LAs and adjuvants are still poorly understood. Regarding the vasoactive potential, LAs as well as the adjuvant epinephrine both have a negative impact on nerve blood flow.9 The question as to whether LAs also have a negative impact on skeletal muscle blood flow has not been adequately addressed yet. Skeletal muscle vasculature is rich in α1 and α2 adrenoreceptors that both mediate a profound vasoconstrictive response and resulting tissue ischemia after activation. Thus, epinephrine as well as the widely used adjuvant clonidine might result in profound muscle vasoconstriction and resulting tissue hypoxemia. These potential alterations in blood might be a potential additional risk factor for muscle damage after peripheral regional anesthesia.
The aim of this study is to evaluate the effect of bupivacaine alone or in combination with the adjuvants epinephrine and clonidine on skeletal muscle blood flow and tissue oximetry using a large-animal model.
Methods
General anesthesia and tissue preparation
Experiments were approved by the local authorities (V 54-19c 2015 (1) MR 20/13 Nr 92/2011, Regional Board of Animal Welfare, Gießen, Germany) and performed in accordance with the current national laws on animal protection. All study results are presented according to the ARRIVE-Guidelines for reporting Animal Studies.10
Twenty-two young female domestic pigs (Sus scrofa domesticus, German Landrace) were used in this experimental study. The animals had a mean age of four months and a mean bodyweight of 30 kg (range: 27–41 kg). In the anesthetized pigs, 40 quadriceps femoris muscle areas were exposed surgically and randomly assigned to one of the four groups. Thus, 10 muscles per group were included in the final data analysis.
Standardized general anesthesia and euthanasia were performed as described in previous studies.5,11,12 In brief, after premedication consisting of diazepam and ketamine, general anesthesia was inducted using propofol (2–3 mg kg−1) and sufentanil 0.2–0.3 mg kg−1. Following endotracheal intubation, general anesthesia was maintained using propofol 0.2 mg kg−1 min−1 and sufentanil 0.5 µg kg−1 min−1. Mechanical ventilation settings were modified to achieve an end tidal CO2 of 35–45 mmHg. Inspiratory oxygen fraction (FiO2) was set to 0.3 to simulate clinical conditions comparable to low-flow oxygen supplementation in spontaneous breathing. An arterial catheter for invasive blood pressure monitoring was inserted via left femoral artery using ultrasound guidance.
To achieve standardized conditions and minimize motion artifacts, pancuronium bromide 0.1 mg kg−1 was administered repeatedly during the study period.
The pharyngeal temperature was monitored to estimate core body temperature and kept at a constant level using warm blankets. All anesthetized animals were placed supine. Quadriceps femoris muscles were exposed surgically without damaging the muscle fascia. For proper positioning of the probe for combined measurement of spectrophotometry and Laser-Doppler flowmetry, a custom-made application device was fixed with sutures on the surrounding tissue to achieve a constant application pressure and measuring volume during the study period.
At the end of the experiments, euthanasia was performed under general anesthesia using potassium chloride (4 mmol kg−1).
Combined tissue spectrophotometry and Laser-Doppler flowmetry
Measurements of muscle blood flow (flow in arbitrary units (AU)), tissue oxygen saturation (SO2 in %) and tissue hemoglobin level (rHb in AU) were performed using Laser spectrophotometry (O2C, Lea Medizintechnik, Gießen, Germany) as described before.5,13 The system and probe were calibrated according to the manufacturer´s protocol.
Tissue blood flow is measured using a Laser Doppler Flowmetry (Figure 1). The tissue spectrophotometry unit calculates hemoglobin content and tissue oximetry. The measuring volume within the tissue is illuminated with coherent Laser light of 500–630 nm wavelength and 30 mW power through a fiber-optic cable. Probe geometry allows for the detection of blood flow at approximately 1–2 mm depth by analyzing backscattered light. By fitting measured spectra with spectra of known tissue saturation values, the percental oxygen saturation of the capillary and post-capillary microvasculature is calculated by the O2C device as SO2. The tissue hemoglobin value (rHb) represents a hemoglobin concentration per tissue volume (displayed as arbitrary unit, AU). It is dependent from microvessel density, venous filling and microvessel shunting. Thus, the rHb is suitable to analyze venous congestion in the measured tissue volume.
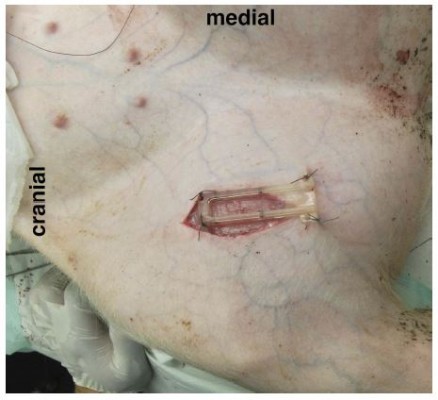
The O2C probe was applied to the exposed muscle tissue with a custom-made application device as shown in Figures 2 and 3 and fixed with sutures for constant application pressure. Probe fixation and measurements were established 10 mins before the intervention to create optimal measurement stability of the system.
After this time span, 1 mL of the randomly assigned test solution was applied to the underlying muscle next to the probe immediately after data were acquired at T0 (timepoint 0 mins).
Study groups
One mL of the test solutions was randomly applied topically to the muscle:
Bupivacaine 0.5% isobaric (group Bupi) (Carbostesin, B. Braun, Melsungen, Germany), Bupivacaine 0.5% isobaric with Epinephrine 1:100.000 (group BupiEpi) (Suprarenin, Sanofi-Aventis, Frankfurt, Germany).
Bupivacaine 0.5% isobaric with 3.75 μg/mL Clonidine (group BupiCloni) (Clonidin ratiopharm, Ratiopharm, Ulm, Germany).
Saline was used as a negative control group: (saline 0.9% isotonic (NaCl, B. Braun, Melsungen, Germany).
All test solutions were prepared fresh immediately before the experiments in a standardized manner using single-dose vials. The concentration of epinephrine and clonidine resemble the highest concentrations of both drugs used as adjuvants to LAs in clinical setting.14
Outcome parameters
The O2C data concerning tissue blood flow (Flow, AU in arbitrary units), tissue oximetry (SO2, in percent) and hemoglobin (rHb, in AU) were recorded at the time points T0, T5, T10, T15, T20, T25, T30, T40, T50 and T60 mins and transferred to an Excel sheet (Microsoft Excel for Mac, Redmond, USA). Systemic hemodynamic parameters as mean arterial blood pressure, heart rate and oxygen saturation were collected at timepoints T10, T30 and T60.
Statistical analysis
Obtained data were analyzed by using SPSS (IBM SPSS version 24.0). Values for SO2 are presented as percentage (%) and values for rHb are given in arbitrary units (AU) according to the manufacturer15 and presented as absolute values at the given time points. Values for blood flow are given as relative flow compared to baseline flow measurement, according to previous studies.5,16,17
Data of relative tissue blood flow (AU), rHb (AU) and SO2 (%) at T0, T30 and T60 of bupivacaine (standard group) were compared with either bupivacaine/clonidine or bupivacaine/epinephrine (experimental groups) using the Mann-Whitney U-test using a hierarchical predefined statistical testing strategy. The saline group served as a negative control group and was compared with bupivacaine only. Statistical tests for differences (two-sided) were deemed significant at a level of p=0.05. Hodges-Lehmann (HL) estimators with 95% confidence intervals (CI) were calculated to analyze the differences of medians between microcirculation parameters (muscle blood flow, SO2 and rHb) between bupivacaine and each group, respectively. Statistical testing for differences (two-sided) was deemed significant at a level of p=0.05. To exclude bias of microcirculatory variations and identify systemic effects of the study drugs, vital parameters between groups at T0, T30 and T60 were compared. HL estimators with 95% CI for heart rate (bpm), mean arterial blood pressure (MAP, mmHg) and SpO2 (%) were calculated between the bupivacaine group and each of the other groups. Differences of less than 15 bpm or heart rate >50, 10 mmHg in MAP or 3% in SpO2 were assumed to be clinically irrelevant.
Results
Vital parameters
Vital parameter data are presented in Table 1. Comparison of HL estimators for mean arterial pressure, heart rate and systemic pulse oximetry revealed no clinically relevant differences between the four groups at T0 (Table 1). At T30 and T60 mins, HL estimators for mean arterial pressure, heart rate and systemic pulse oximetry showed no clinically relevant differences between the four groups.
 |
Table 1 Vital parameters |
Microcirculation
Data of relative muscle flow parameters are presented in Table 2. The bupivacaine group showed no significant difference in relative flow parameters compared with the saline group as control group at T30 (p=0.853) and T60 (p=0.218). However, a significant decrease in relative blood flow was revealed when bupivacaine with clonidine was compared with bupivacaine alone at T30 (p=0.011) and T60 (p=0.043). Comparison of bupivacaine with bupivacaine/epinephrine showed a significant decrease in relative flow at T30 (p=0.03) and T60 (p=0.002). Analysis of HL estimators between bupivacaine and BupiCloni or BupiEpi revealed a relevant difference of relative muscle blood flow reductions at T30 and T60.
 |
Table 2 Relative muscle blood flow |
Tissue oximetry
Data for SO2 measurements are presented in Table 3. The Mann-Whitney test was performed for T0, T30 and T60 comparing each group with bupivacaine. At T0, no statistically significant differences were revealed between the four groups. Statistical analyses show significant differences comparing bupivacaine with saline at T 30 (p=0.01) and T60 (p=0.01). Comparison of bupivacaine with bupivacaine/clonidine showed no significant differences at T0 (p=0.247), T30 (p=0.315) and T60 (p=0.912). Comparison of bupivacaine with bupivacaine/epinephrine revealed no statistically significant differences at T0 (p=0.529), T30 (p=0.123) and T60 (p=0.280). HL estimators (and 95% CI) for comparisons between bupivacaine group and the three other groups were calculated for T0, T30 and T60 and are presented in Table 3.
 |
Table 3 Tissue oximetry |
Hemoglobin content
Analysis of relative hemoglobin content as a parameter of venous congestion is presented in Table 4. No statistically significant differences of rHb could be detected comparing bupivacaine with saline at T0 (p=0.684), T30 (p=0.739) and T60 (p=0.631). Moreover, comparison of rHb of the bupivacaine group with the bupivacaine/clonidine group revealed no statistically significant differences at T0 (p=0.063), T30 (p=0.280) and T60 (p=0.393). Comparison of the bupivacaine group with the bupivacaine/epinephrine group showed no significant differences at T0 (p=0.912), T30 (p=0.853) and T60 (p=0.631). Results of HL estimator and 95% CI were calculated for T0, T30 and T60 as shown.
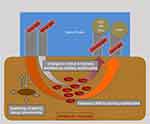 |
Figure 1 Schematic illustration of O2C device (SO2, tissue oxygen saturation; rHb, tissue hemoglobin). |
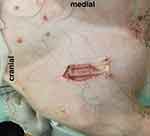 |
Figure 2 Mounting device (O2C probe removed). |
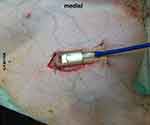 |
Figure 3 Mounted probe in situ. |
 |
Table 4 Relative hemoglobin content |
Discussion
Our experimental data showed that the application of bupivacaine results in a significant reduction of measured muscle blood flow at T30 and T60 (Table 4) when bupivacaine was compared to the saline control group. Interestingly, the addition of clonidine as well as epinephrine to bupivacaine results in pronounced additional blood flow reductions.
Compared to saline, the topical application of bupivacaine results in profound reductions of tissue oximetry values at T30 as well as at T60. However, the addition of clonidine or epinephrine did not show any difference (as an additional risk factor for tissue ischemia) regarding tissue oximetry compared to bupivacaine alone.
To our knowledge, this is the first study investigating the effect of bupivacaine on skeletal muscle microcirculation. We showed a pronounced reduction of muscle perfusion which resulted in consecutive reductions of tissue oximetry within the measured tissue volume. A previous study by Palmer et al. showed no effect of lidocaine 10 mg mL−1 on muscle perfusion in an experimental model in rats using the radiolabeled microsphere technique.18 Interestingly, in the same study by Palmer, the addition of epinephrine (10 µg mL−1) to lidocaine did result in only modest reductions of muscle blood flow. A previous study by Johns et al. found a dose-dependent vasoconstrictive effect on rat cremaster arterioles.19
Epinephrine is used as an adjuvant for LAs to reduce systemic absorption and toxic effects. A shortened nerve block onset time and prolonged block duration is assumed when epinephrine is used.14 It prolongs nerve block duration of LAs due to its reduction of local tissue clearance and enhanced concentration of LA in the perineural tissue.9,20,21 Our data is in contrast to the Palmer study, as we were able to demonstrate a significant reduction of muscle blood flow compared to saline when bupivacaine alone or in combination with epinephrine or clonidine was used. Clonidine is commonly used as an adjuvant to LA in regional anesthesia.14 Underlying mechanisms are still unknown. Interestingly, due to α2-adrenergic effects of clonidine, one might assume a resulting vasodilatation with the increase of tissue blood flow. Contrary to a previous study by our group5 investigating the effect of adjuvants on nerve blood flow showing no effect of clonidine when added to bupivacaine, the addition of clonidine to bupivacaine did result in an additional decrease of muscle tissue blood flow in this study. The extent of additional decrease of tissue perfusion by adding clonidine is comparable with the effect of addition of epinephrine to bupivacaine in the BupiEpi group in our study. These reductions of muscle blood flow resulted in profound reductions of skeletal muscle tissue oximetry in all three groups containing bupivacaine. Between these three groups, there was no difference in the extent of ischemia, even despite a numeric but not statistically significant difference of the BupiCloni group compared with bupivacaine alone or in combination with epinephrine. The microcirculation of skeletal muscle is regulated in a complex system comprising systemic (mediated by hormonal or nervous effects) as well as local factors (myogenic, metabolic or endothelial mechanism) that differ in resting and activated muscles.22
In general, arterial (but not venous) branches of microcirculation in skeletal muscles are supplied by adrenergic sympathetic nervous fibers with α1 and 2 as well as beta-2 receptors. In previous studies, the postsynaptic α2 adrenoreceptor activation resulted in profound reductions of skeletal muscle perfusion.23,24
This might be the pharmacological explanation of reductions of tissue perfusion in the BupiCloni group. The clinical impact of our results is unclear as we were only able to measure short-term effects of bupivacaine in our study. However, the ischemic effect of bupivacaine alone or in combination with the adjuvants epinephrine or clonidine might be an additional risk factor to LA-induced myotoxicity after peripheral nerve blocks. LA-induced myotoxicity is induced by bupivacaine-related early and late aberrations in cytoplasmatic Ca2+ homeostasis.7,25 The clinical impact of LA-induced myotoxicity is controversially discussed. Clinical relevant myopathy and myonecrosis following continuous peripheral nerve blocks, infiltration of wound margins and peri- and retrobulbar blocks have been described.2 There are several reports of myotoxicity due to LAs. Zink et al. reported that when bupivacaine and ropivacaine were continuously administered through femoral nerve catheters over six hours, both caused irreversible skeletal damage.3 Neal et al. reported that three individuals who received adductor canal block with lidocaine or mepivacaine bolus followed by ropivacaine for continuous infusion developed progressive weakness of quadriceps muscles.26 Overall, the clinical impact of LA myotoxicity has been controversially debated. However, there is good experimental evidence that LAs caused lesions in skeletal muscle tissue. In a recent systematic review by Hussain et al., the authors concluded that further research needs to be performed to rule out the effect of adjuvants to LAs on myotoxicity.25 We might speculate that combined injury by profound ischemia in addition to the myotoxicity of the LAs used might result in more severe muscle damage.
Limitations
This experimental study has several limitations that should be addressed. Firstly, the differences between the results in this animal experimental study might not be transferred to clinical conditions, due to differences in muscle blood flow and regulative mechanisms. Vascular tone might be influenced by general anesthesia, surgical preparation. Changes in blood pressure, systemic oxygen saturation and end-tidal carbon dioxide might influence skeletal muscle blood flow. We aimed to control this bias by invasive blood pressure monitoring, controlled mechanical ventilation with fixed inspired oxygen fraction and the maintenance of normocapnia with the use of capnometry. Measurements were analyzed as individual cases despite performing up to two measurements (left and right) per animal. This approach is similar to other published studies by our group and others with the aim to reduce animal numbers.
In conclusion, our study demonstrated profound reductions of skeletal muscle blood flow as well as tissue oximetry after topical application of bupivacaine alone in comparison with saline solution as control. The addition of epinephrine or clonidine both resulted in additional reductions of local muscle blood flow but without further reductions of tissue oximetry values.
Abbreviations
AU, arbitrary unit; Ca2+, Calcium (ionized); LA, local anesthetic; MAP, mean arterial blood pressure; n, number; rel, relative; rHb, relative tissue hemoglobin; SO2, tissue oxygen saturation; SpO2, systemic arterial oxygen saturation; O2C, “oxygen to see” (Proprietary name of LEA company, Giessen, Germany).
Data sharing statement
Full raw data can be requested from the corresponding author.
Ethical approval
Experiments were approved by the local authorities (V 54-19c 2015 (1) MR 20/13 Nr 92/2011), Regional Board of Animal Welfare, Gießen, Germany.
Acknowledgment
We thank Andreas Gockel for his outstanding performance in support of data acquisition in our animal lab. This study was performed with institutional funding.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Thomas Wiesmann reports personal fees from B. Braun and from Pajunk, outside the submitted work. Hinnerk Wulf reports speaker fees from Syntetica, B. Braun, Teleflex and Vygon. Thorsten Steinfeldt reports speaker fees from B. Braun, Teleflex and Vygon. The authors report no other conflicts of interest in this work.
References
1. Zink W, Seif C, Bohl JR, et al. The acute myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blockades. Anesth Analg. 2003;97(4):1173–1179. table of contents. doi:10.1213/01.ane.0000080610.14265.c8
2. Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29(4):333–340.
3. Zink W, Bohl JR, Hacke N, Sinner B, Martin E, Graf BM. The long term myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blocks. Anesth Analg. 2005;101(2):548–554. table of contents. doi:10.1213/01.ANE.0000155956.59842.0A
4. Wiesmann T, Steinfeldt T, Exner M, et al. Intraneural injection of a test dose of local anesthetic in peripheral nerves – does it induce histological changes in nerve tissue? Acta Anaesthesiol Scand. 2017;61(1):91–98. doi:10.1111/aas.12825
5. Wiesmann T, Müller S, Müller HH, Wulf H, Steinfeldt T. Effect of bupivacaine and adjuvant drugs for regional anesthesia on nerve tissue oximetry and nerve blood flow. J Pain Res. 2018;11:227–235. doi:10.2147/JPR.S152230
6. Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59(10):727–736.
7. Benoit PW, Yagiela A, Fort NF. Pharmacologic correlation between local anesthetic-induced myotoxicity and disturbances of intracellular calcium distribution. Toxicol Appl Pharmacol. 1980;52(2):187–198. doi:10.1016/0041-008x(80)90105-2
8. Irwin W, Fontaine E, Agnolucci L, et al. Bupivacaine myotoxicity is mediated by mitochondria. J Biol Chem. 2002;277(14):12221–12227. doi:10.1074/jbc.M108938200
9. Neal JM. Effects of epinephrine in local anesthetics on the central and peripheral nervous systems: neurotoxicity and neural blood flow. Reg Anesth Pain Med. 2003;28(2):124–134. doi:10.1053/rapm.2003.50024
10. Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol. 2012;41(1):27–31. doi:10.1111/j.1939-165X.2012.00418.x
11. Steinfeldt T, Graf J, Schneider J, et al. Histological consequences of needle-nerve contact following nerve stimulation in a pig model. Anesthesiol Res Pract. 2011;2011:591851.
12. Steinfeldt T, Wiesmann T, Nimphius W, et al. Perineural hematoma may result in nerve inflammation and myelin damage. Reg Anesth Pain Med. 2014;39(6):513–519. doi:10.1097/AAP.0000000000000170
13. Wiesmann T, Freitag D, Dersch W, et al. Dantrolene versus amiodarone for cardiopulmonary resuscitation: a randomized, double-blinded experimental study. Sci Rep. 2017;7:40875. doi:10.1038/srep40875
14. Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015;10(9):e0137312. doi:10.1371/journal.pone.0137312
15. Krug A. Microcirculation and oxygen supply: method of so-called O2C. Phebologie. 2007;36:300–312.
16. Bouaziz H, Iohom G, Estèbe JP, Campana WM, Myers RR. Effects of levobupivacaine and ropivacaine on rat sciatic nerve blood flow. Br J Anaesth. 2005;95(5):696–700. doi:10.1093/bja/aei242
17. Myers RR, Heckman HM. Effects of local anesthesia on nerve blood flow: studies using lidocaine with and without epinephrine. Anesthesiology. 1989;71(5):757–762. doi:10.1097/00000542-198911000-00021
18. Palmer GM, Cairns BE, Berkes SL, Dunning PS, Taylor GA, Berde CB. The effects of lidocaine and adrenergic agonists on rat sciatic nerve and skeletal muscle blood flow in vivo. Anesth Analg. 2002;95(4):1080–1086. table of contents. doi:10.1097/00000539-200210000-00054
19. Johns RA, DiFazio CA, Longnecker DE. Lidocaine constricts or dilates rat arterioles in a dose-dependent manner. Anesthesiology. 1985;62(2):141–144. doi:10.1097/00000542-198502000-00008
20. Niemi G. Advantages and disadvantages of adrenaline in regional anaesthesia. Best Pract Res Clin Anaesthesiol. 2005;19(2):229–245.
21. Bernards CM, Kopacz DJ. Effect of epinephrine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology. 1999;91(4):962–968. doi:10.1097/00000542-199910000-00015
22. Hudlicka O. Microcirculation in skeletal muscle. Muscles Ligaments Tendons J. 2011;1(1):3–11.
23. Jarajapu YP, Coats P, McGrath JC, Hillier C, MacDonald A. Functional characterization of alpha(1)-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br J Pharmacol. 2001;133(5):679–686. doi:10.1038/sj.bjp.0704130
24. Moore AW, Jackson WF, Segal SS. Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol. 2010;588(Pt 21):4261–4274. doi:10.1113/jphysiol.2010.194993
25. Hussain N, McCartney CJL, Neal JM, Chippor J, Banfield L, Abdallah FW. Local anaesthetic-induced myotoxicity in regional anaesthesia: a systematic review and empirical analysis. Br J Anaesth. 2018;121(4):822–841. doi:10.1016/j.bja.2018.05.076
26. Neal JM, Salinas FV, Choi DS. Local anesthetic-induced myotoxicity after continuous adductor canal block. Reg Anesth Pain Med. 2016;41(6):723–727. doi:10.1097/AAP.0000000000000466
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
