Back to Journals » Clinical Interventions in Aging » Volume 10
Effect of aging on oral and swallowing function after meal consumption
Authors Tetsuya Hiramatsu, Kataoka H, Osaki M, Hagino H
Received 1 October 2014
Accepted for publication 12 November 2014
Published 9 January 2015 Volume 2015:10 Pages 229—235
DOI https://doi.org/10.2147/CIA.S75211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Tetsuya Hiramatsu,1,2 Hideyuki Kataoka,3 Mari Osaki,4 Hiroshi Hagino3,4
1Department of Speech Pathology and Audiology, Matsue Co-medical College, Matsue, Japan; 2Graduate School of Medical Sciences, Tottori University, 3School of Health Science, Faculty of Medicine, Tottori University, 4Rehabilitation Division, Tottori University Hospital, Yonago, Japan
Background/purpose: Dysphagia may worsen due to fatigue of the infrahyoid and suprahyoid muscle groups as a result of repetitive swallowing during a meal. We investigated the hypothesis that meal consumption may reduce tongue strength and endurance in older adults (OAs).
Methods: Tongue–palate pressure, oral diadochokinesis, repetitive saliva swallowing, and surface electromyography activity before and after a meal were measured in 23 young adults (YAs) and 23 OA volunteers.
Results: There was a statistically significant difference in both tongue pressure and the number of voluntary swallows between YAs and OAs. Peak tongue pressure was significantly lower in OAs than YAs both before and after meal consumption. The most notable finding was that the first time interval (the time from test initiation to the beginning of the first swallow) was prolonged after meal consumption only in OAs, whereas the first time interval showed no difference between YAs and OAs before meal consumption with reference to the repetitive saliva swallowing test. The initiation of swallowing was prolonged by both meal consumption and aging; there was a significant interaction between these two factors. The number of repetitions of the monosyllable/pa/was statistically similar between YAs and OAs before meal consumption, but it was significantly lower in OAs after meal consumption.
Conclusion: Aging leads to declining tongue pressure and motor function of the lips. It is possible that swallowing function declines in older individuals when meal consumption is prolonged, especially at the end of mealtime, as a result of their efforts in mastication and swallowing.
Keywords: normal adults, dysphagia, fatigue, elderly
Introduction
Swallowing is a complex neuromuscular activity that consists of oral, pharyngeal, and esophageal phases, and involves the coordinated function of many muscles. Suprahyoid muscles consist of mylohyoid, geniohyoid, stylohyoid, and anterior belly of the digastric. The thyrohyoid muscle decreases the distance between the hyoid bone and the thyroid cartilage. These muscles work together to raise the larynx.1 From an anatomic and physiologic perspective, aging organ systems are usually slower and exhibit diminished strength, stability, coordination, and endurance.2 Thus, it is thought that elderly individuals tend to need more time to consume food and the efficiency of the muscles involved in swallowing is reduced and more fatigable. An increased prevalence of aspiration and pneumonia is associated with fatigue of the infrahyoid and suprahyoid muscle groups with repetitive swallowing during meals. The duration of swallows and total drinking time are significantly increased in the elderly.3 Elderly individuals with swallowing disorders are advised to eat slowly. They should keep eating for more than 30 minutes with the help of a meal assistant to obtain the necessary energy from food. Longer meal durations may cause muscle fatigue, thereby possibly raising the risk of aspiration. Relatively few studies have investigated the effect of meal consumption on oral and swallowing function in elderly individuals. Kays et al were the first to study the tongue’s performance before and after a meal.4 In addition, there was a trend toward longer meal duration in older adults (OAs). Therefore, we asked each subject to spend 30 minutes in meal consumption in order to more specifically evaluate fatigue of the muscles involved in mastication and swallowing associated with food consumption. The purpose of this study was to examine the effort required for swallowing during meal consumption in healthy OAs versus young adults (YAs) through pre-meal and post-meal electromyography (EMG) and evaluation of tongue pressure, oral diadochokinesis, and repetitive saliva swallowing before and after a meal lasting 30 minutes.
Subjects and methods
Subjects
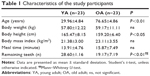
The study participants consisted of 46 healthy adults who provided informed consent to participate in our research study. YA volunteers were recruited by placing an advertisement for vocational school students. Healthy OA volunteers were recruited from the membership of local clubs for healthy elderly individuals. There were 23 healthy YA volunteers (13 men and 10 women) ranging in age from 21 years to 38 years (mean, 29.96±4.84 years). There were 23 healthy OA volunteers (14 men and 9 women) over 70 years of age (mean, 76.65±4.86 years; range, 70–84 years) recruited from the community (Table 1). There were no significant differences between the two groups except for age, height, and number of remaining teeth.
At baseline, a self-administered questionnaire was administered to all the participants. The questionnaire included items such as a self-evaluation of general and oral health status, whether one was under a doctor’s care, current medications, number of remaining teeth, meal duration, oral hygiene behaviors, and self-reported symptoms of oral dysfunction such as a sensation of xerostomia, dysphagia, or coughing while eating.
Subjects with cerebrovascular disease, reflux esophagitis, aspiration pneumonia, weight loss, aphagia, odynophagia, laryngeal tightness while swallowing, difficulties in swallowing solids or liquids, prolonged meal duration, craniocervical pain, episodes of aspiration during consumption of fluids, or subjective sensation of xerostomia in their daily lives or severe xerostomia identified during intraoral observation were excluded. If subjects usually wear dentures in their daily life, we asked them to wear them during the study.
Methods
The order of oral function evaluation is outlined in Table 2.
  | Table 2 Evaluation of oral function |
Tongue–palate pressure
Swallowing tongue pressures were measured with the JMS tongue pressure manometer (JMS Co. Ltd, Tokyo, Japan). Measurements of maximum tongue pressure against the hard palate were performed using a balloon probe. Subjects were asked to press their tongue against the roof of their mouth as hard as possible (Figure 1). Pressure was measured in kilopascals using a digital voltmeter. The bulb and pressure sensor were constructed of flexible plastic; the pressure point was marked during the first measurement so that maximum tongue pressure was measured at the same location before and after the meal.
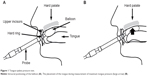  | Figure 1 Tongue–palate pressure test. |
Oral diadochokinesis
To measure oral diadochokinesis, a microphone was positioned in front of the mouth of the subject who was in a seated position. Participants were given instructions to repeat each of the following syllables /pa/, /ta/, or /ka/ as quickly as possible for 5 seconds, until the bell signaled the end of the measurement.
Pronouncing the syllables /pa/, /ta/, or /ka/ involves the use of the front (lips), middle (tip of the tongue), or back of the mouth (posterior tongue), respectively. Monosyllabic emissions of /pa/, /ta/, or /ka/ were analyzed using a device used for counting (Kenko-kun; Takei Scientific Instruments, Niigata, Japan). The total number of pronounced syllables was automatically counted by Kenko-kun. The repetition speed was calculated as the number of repetitions per second. Evaluation of the diadochokinetic rate was performed as part of an oral motor skill assessment.
Repetitive saliva swallowing test
The repetitive saliva swallowing test (RSST), which involves more than three cycles of swallowing within 30 seconds,5 was conducted to evaluate the pharyngeal phase of swallowing under wet conditions in the mouth using Kenko-kun. The subject was in a seated position during the test, which lasted 30 seconds. The subject was instructed to start swallowing upon hearing a beeping sound. The examiner timed how long it took for each subject to make three separate palpable oral and pharyngeal swallowing motions, respectively. The latency of the swallowing reflex was calculated from the total measured duration. The frequency of pharyngeal swallowing motions during the 30-second period and the time lapse between the first and third pharyngeal swallowing acts, respectively, in each interval were measured. Subjects were asked to swallow saliva as many times as possible in a seated position, during which the number of elevations of the hyoid bone and the laryngeal prominence was counted. The onset of each swallow was judged based on palpation of the laryngeal prominence by the examiner’s finger.
The first time interval was defined as the time from the starting beep to the evocation of the first swallow. The second and third time intervals were defined as the time interval between the first and second swallow, and the second and third swallow, respectively.
Surface EMG
EMG was performed using a surface EMG apparatus (KM-104FR; Inter-Riha Co. Ltd, Tokyo, Japan). EMG of the suprahyoid muscles was performed in a seated position. Two surface electrodes separated by a distance of 10 mm were attached to the skin beneath the chin to the right of the midline to record submental myoelectrical activity over the platysma. A third electrode was affixed to the elbow as the ground electrode. Electrical impedance at the sites of electrode contact was reduced by light scrubbing with alcohol gauze pads and application of an electrode gel. Muscle activity was calculated by summing the integral of the reflected EMG waves. Muscle activity and fatigue were evaluated by surface EMG of the suprahyoid muscles with the head raised on an AIREX® fitness mat (Airex AG, Sins, Switzerland). Each subject was asked to sustain a raised head position by looking at the toes without raising the shoulders off the ground.6 The mean submental surface EMG frequency was measured while the position was maintained for 4 seconds. Subjects were instructed to raise their heads from the same supine position before and after a meal. The sampling rate was 1,000 Hz. Raw signals were integrated and rectified. The maximal voluntary contraction (MVC) was defined as suprahyoid muscle activity for 2 seconds of the 4 seconds of pre-meal EMG frequency measurement in the raised head position. The muscle activity to MVC ratio was presented as a percentage of MVC (%MVC).
Water swallowing test
Subjects were asked to sit on the chair while 3 mL of cold water was poured into their mouths and retained for a few seconds. They were then asked to swallow all the water at once. Muscle activity was recorded during the swallow.
Before the swallowing study, surface EMG signals were recorded for 0.5 seconds at rest when the muscle was totally relaxed. The average amplitude was used as a baseline. Surface EMG signals were monitored during the entire swallowing examination. The onset of swallowing was defined as the time point at which the amplitude of muscle activation was two standard deviations higher than baseline. Muscle activity was based on the mathematical integral of the EMG amplitude during the 2 seconds after the subject began swallowing 3 mL of cold water.
Meal
The meal used in this study was typical for the participants (both YAs and OAs). It consisted of foods that are commonly consumed at lunch or dinner in Japan. The meal was administered on a tray. Subjects ate a bowl of rice topped with chicken and eggs for 30 minutes. Subjects were seated at a table and instructed to take all 30 minutes to eat as naturally as possible without talking or resting.
The meal duration of 30 minutes was selected to simulate high-intensity eating during which mastication and swallowing were continuously repeated, similar to the situation when a patient eats in a continuous fashion with the support of a meal assistant. We ensured that subjects did not stop or take a break in chewing or swallowing.
Data analysis
Pre-meal and post-meal mean values were compared using a paired Student’s t-test if the values were normally distributed or the Wilcoxon rank sum test if the data were not normally distributed. Mean values of the two groups were compared using Student’s t-test if the values were normally distributed or the Mann–Whitney or Wilcoxon rank sum test otherwise. Data on latency of the swallowing reflex were analyzed using repeated three-way analysis of variance (ANOVA) with age, meal, and number of repeated saliva swallows and their interaction terms. Multiple comparisons were performed using Tukey’s test. The level of significance was defined as P<0.05. Statistical analysis was conducted using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
All protocols were approved by the local ethics committees of the Faculty of Medicine, Tottori University (No 1490).
Results
Tongue pressure
Pre-meal and post-meal tongue pressures in YAs were significantly higher than in OAs. Pre-meal and post-meal tongue pressures were not significantly different within each age group (Table 3).
Repetitive saliva swallowing test
The numbers of swallows are shown in Table 3. The number of swallows during 30 seconds was significantly higher in YAs than OAs both pre-meal (P<0.01) and post-meal (P<0.001). Although the number of swallows before and after meal consumption was similar in YAs, OAs had a significantly lower number of post-meal swallows (P<0.05).
RSST results are shown in Figure 2. The pre-meal first time interval was almost the same in YAs and OAs, but the second and third time intervals in OAs were longer than in YAs. The first time interval post-meal was longer than the pre-meal value only in OAs. The first time interval post-meal of OAs was significantly longer than that of YAs.
ANOVA showed that the time interval after the meal was longer compared to before the meal (P<0.05), and was longer in OAs compared to YAs (P<0.001).
Oral diadochokinesis
Significant post-meal differences were observed between YAs and OAs (P<0.05) in the oral diadochokinetic rate for repetition of the monosyllable /pa/, but not pre-meal. For the monosyllable /ta/, there were significant pre-meal (P<0.01) and post-meal (P<0.01) differences in the oral diadochokinetic rate between YAs and OAs. For the monosyllable /ka/, there were significant differences in the oral diadochokinetic rate between YAs and OAs pre-meal (P<0.01) and post-meal (P<0.01). Within each age group, there was no significant difference in the oral diadochokinetic rate before and after the meal.
Surface EMG
In YAs, %MVC for swallowing 3 mL of cold water was 114.19%±60.98% pre-meal and 120.79%±70.15% post-meal. In OAs, it was 114.48%±55.08% and 112.90%±57.58%, respectively. Differences between pre-meal and post-meal and between the groups were not statistically significant.
The duration of muscle activity while swallowing 3 mL of cold water as evaluated by EMG was 3.55±0.89 seconds pre-meal and 3.34±0.71 seconds post-meal in YAs and 3.57±0.76 seconds and 3.51±1.18 seconds in OAs, respectively, showing no significant differences.
In YAs, the mean submental surface EMG frequency was 120.96±23.97 Hz pre-meal and 118.12±24.13 Hz post-meal, compared to 109.30±20.45 Hz and 109.35±20.09 Hz, respectively, in OAs. Differences between pre-meal and post-meal and between the groups were not statistically significant.
Discussion
In the present study, we evaluated tongue–palate pressures, RSST, oral diadochokinesis, and surface EMG activity to assess the effects of muscle fatigue on both oral and pharyngeal swallowing function associated with meal consumption.
As shown in Table 3, there was a statistically significant difference in both tongue pressure and the number of voluntary swallows between YAs and OAs. Peak tongue pressures were significantly lower in OAs than YAs both before and after meal consumption. Maintaining sufficient tongue pressure might be associated with the ability to smoothly form a bolus and better swallowing function in OAs. In the oral phase of ingestion, food is brought into the mouth, chewed, and combined with saliva to form a bolus that is moved to the back of the oral cavity and prepared for swallowing.7 Based on our findings, we speculate that lingual resistance exercises may be a beneficial treatment strategy for frail aged patients with lingual weakness and swallowing disability. However, the relationship between tongue pressure and swallowing function needs to be studied further with videofluoroscopic swallowing examinations.
The most notable finding was that the first time interval was prolonged after meal consumption only in OAs, whereas the first time interval showed no difference between YAs and OAs before meal consumption with reference to RSST. The elderly have decreased swallowing function, especially just after eating, according to the meal consumption being prolonged. Ligamentous laxity, reduced muscle tone in the pharynx and esophagus, and increased duration of swallowing are recognized as physiological changes due to aging.8 In elderly individuals, muscle wasting and weakness can occur.
Aging is associated with decreases in total muscle cross-sectional area, amounting to approximately 40% between the ages of 20 years and 80 years.9 These changes result from a loss of functioning motor units.10
The number of motor units declines with increasing age, especially after mid-life, so that elderly subjects often have less than one-half the number of functioning motor units that they had in youth, despite good strength.11 These changes likely increase the time interval, especially after meal consumption in elderly individuals.
In the oral phase of ingestion, mastication, tongue mobility, and lip closure are important parts of forming a bolus.7 These functions seem to deteriorate with age.12 In this study, as shown in Figure 3, the number of repetitions of the monosyllable /pa/was similar between YAs and OAs before meal consumption, but OAs had significantly fewer repetitions of the monosyllable /pa/ than YAs after meal consumption. Skeletal muscles contain two main types of fibers: slow-twitch fibers (Type I) and fast-twitch fibers (Type II). Type II fibers are further divided in two groups, Type IIa and IIb. Type I fibers are resistant to fatigue and can work for long periods of time. Type IIa fibers are intermediate fast-twitch fibers. They can work for no more than 30 minutes, and they fatigue faster than Type I fibers. Type IIb fibers are the classic fast-twitch fibers. They fire faster than Type IIa fibers but are able to work only for a few minutes. YAs had a higher number of repetitions of the monosyllables /ta/ and /ka/ than OAs after meal consumption. Repetition of the monosyllable /pa/ was limited by meal consumption in YAs. There is a relatively high concentration of Type I (28%) and Type IIa (72%) fibers in both the superior and inferior portions of the orbicularis oris. There is a blend of fatigue-resistant Type I fibers and relatively faster-contracting Type IIa fibers.13 The monosyllable /pa/ requires repetitive opening of the lip, which is composed of the superior and inferior portions of the orbicularis oris. Burkhead et al demonstrated that the more rapidly contracting, larger diameter Type IIb fibers are concentrated toward the base of the tongue and in the pharyngeal constrictors, which produce rapid, more forceful actions during swallowing.14 The monosyllable /ka/ requires repetitive elevation of the posterior tongue. Many of the shapes adopted by the tongue in speaking are also used in eating.15 Since the meal in the current study included both hard and soft foods, the tongue had to change its shape in order to adapt to the type of food.
However, the posterior tongue contains a higher percentage of slow-twitch muscle fibers, a pattern that may expand to the entire tongue with aging.9 Even if subjects continue to eat a meal for 30 minutes and perform deglutition repeatedly, it is possible that the motor function of the posterior tongue would be less affected than the orbicularis oris. Although we did not examine the masseter muscle in this study, it is possible that the lower jaw may play a role in these articulations.
Surface EMG studies of pharyngeal swallowing are generally performed to evaluate the pattern of activity in the submental muscles.16 Mean %MVC in the surface EMGs was over 100% during pre-meal and post-meal swallowing. Suprahyoid muscle activity might not reach its maximum since other muscles such as the sternocleidomastoid were active when MVC values were recorded in the raised head position; however, %MVC during swallowing represents relative muscle activity. The findings suggest that relative muscle activity is not affected by a meal lasting 30 minutes or aging, since there were no pre-meal versus post-meal or YA versus OA differences in %MVC. The meal in the current study may not have induced suprahyoid muscle fatigue since the suprahyoid surface EMG frequency did not change after meal consumption. These results suppose that the suprahyoid muscles were not influenced by prolonged mastication and swallowing during a meal lasting 30 minutes, but rather that they would continue to function normally once pharyngeal swallowing occurs. Further studies might be needed to evaluate suprahyoid muscle fatigue with aging in studies involving more mastication.
Conclusion
This study showed that the initiation of swallowing was prolonged by both meal consumption and aging, and there was a significant interaction between these two factors. Aging leads to reduced tongue pressure and motor function of the lips. It is possible that the swallowing function declines in older individuals when meal consumption is prolonged, especially at the end of mealtime, as a result of their efforts in mastication and swallowing.
Disclosure
We have no conflicts of interest to declare.
References
Burnett TA, Mann EA, Cornell SA, Ludlow CL. Laryngeal elevation achieved by neuromuscular stimulation at rest. J Appl Physiol. 2003; 94:128–134. | ||
Chodzko-Zajko WI, Ringel RL. Physiological aspects of aging. J Voice. 1987;1:18–26. | ||
Vaiman M, Gabriel C, Eviatar E, Segal S. Surface electromyography of continuous drinking in healthy adults. Laryngoscope. 2005;115: 68–73. | ||
Kays SA, Hind JA, Gangnon RE, Robbins J. Effects of dining on tongue endurance and swallowing-related outcomes. J Speech Lang Hear Res. 2010;53:898–907. | ||
Tamura F, Mizukami M, Ayano R, Mukai Y. Analysis of feeding function and jaw stability in bedridden elderly. Dysphagia. 2002;17:235–241. | ||
Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272:1518–1522. | ||
Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Austin, TX: Pro-Ed; 1998. | ||
Sheth N, Diner WC. Swallowing problems in the elderly. Dysphagia. 1988;2:209–215. | ||
Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. | ||
Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–182. | ||
Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry. 1972;35:845–852. | ||
Fucile S, Wright PM, Chan I, Yee S, Langlais ME, Gisel EG. Functional oral motor skills: do they change with age? Dysphagia. 1998;13:195–201. | ||
Stål P, Eriksson PO, Eriksson A, Thornell LE. Enzyme-histochemical and morphological characteristics of muscle fibre types in the human buccinator and orbicularis oris. Arch Oral Biol. 1990;35:449–458. | ||
Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22:251–265. | ||
Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Boil Med. 2003;14:413–429. | ||
Vaiman M, Eviatar E, Segal S. Evaluation of normal deglutition with the help of rectified surface electromyography records. Dysphagia. 2004;19:125–132. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.