Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Drug-induced skin reactions: a 2-year study
Authors Farshchian M, Ansar A, Zamanian A, Rahmatpour-Rokni G, Kimyai-Asadi A, Farshchian M
Received 15 October 2014
Accepted for publication 24 November 2014
Published 9 February 2015 Volume 2015:8 Pages 53—56
DOI https://doi.org/10.2147/CCID.S75849
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Mahmood Farshchian,1 Akram Ansar,1 Abbas Zamanian,2 Ghasem Rahmatpour-Rokni,1 Arash Kimyai-Asadi,3 Mehdi Farshchian1,4
1Psoriasis Research Center, Department of Dermatology, Farshchian Hospital, Hamadan University of Medical Sciences, Hamedan, Iran; 2Department of Dermatology, Iran University of Medical Sciences, Tehran, Iran; 3Derm Surgery Associates, Houston, TX, USA; 4Department of Dermatology, University of Turku and Turku University Hospital, Turku, Finland
Background: The aim of this study was to analyze the clinical characteristics of patients with adverse cutaneous drug reactions, which occur when a medicinal product results in cutaneous morbidity.
Methods: The study included 308 patients who were diagnosed as having an adverse cutaneous drug reaction during the study period (2007–2009). In 84 cases, histopathologic examination of skin biopsies were also performed.
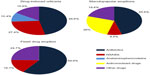
Results: Patients with drug reactions were found to be more commonly female (63%) than male (37%). Beta-lactam antibiotics were found to be the most frequent cause of adverse cutaneous drug reactions (42.7%), followed by non-steroidal anti-inflammatory drugs (16.5%). Acute urticaria was the most common clinical presentation (59.2%) followed by fixed drug eruptions (18.5%), and maculopapular eruptions (14.9%).
Conclusion: Adverse cutaneous drug reactions in our study population were mainly induced by beta-lactam antibiotics and non-steroidal anti-inflammatory drugs. The most common forms of cutaneous adverse drug reactions were found to be acute urticaria, fixed drug eruptions, and maculopapular rashes.
Keywords: adverse drug reaction, acute urticaria, exanthematous eruption
Introduction
Adverse drug reactions (ADRs) are undesirable and typically unanticipated reactions independent of the intended therapeutic purpose of a medication,1 that may result in significant morbidity and even mortality. Cutaneous reactions are the most common form of ADRs,2 occurring in 2%–3% of inpatient and in approximately 2% of outpatient patients referred for dermatologic evaluation; approximately 2% of ADRs are considered severe or fatal.3,4 Drug reactions are more common in women, and increase with age and the number of medications used.5
ADRs may be either immunologic (ie, drug allergy) or non-immunologic (ie, drug intolerance), with drug allergies estimated to account for 6%–10% of all ADRs, and drug intolerance accounting for the remaining 90%–94%.6 Cutaneous ADRs produce a wide range of clinical manifestations such as pruritus, maculopapular eruptions, urticaria, angioedema, phototoxic and photo allergic reactions, fixed drug reactions, erythema multiforme, vesiculobullous reactions (eg, Stevens–Johnson syndrome and toxic epidermal necrolysis), exfoliative dermatitis, acute generalized exanthematous pustulosis, and serum sickness.7,8 Whereas maculopapular rashes and urticaria are among the most common cutaneous drug reactions,9 anaphylaxis, Stevens–Johnson syndrome, and toxic epidermal necrolysis may result in mortality.10
The purpose of this study was to examine the clinical characteristics and purported etiologic agents for ADRs in our patient population.
Materials and methods
A descriptive, prospective case-series study was performed during 2007–2009. Three hundred and eight consecutive inpatient and outpatient subjects with a diagnosis of ADR referred to the dermatology service of Sina hospital in Hamadan, Iran were enrolled in the study. All patients suspected of having an ADR were clinically evaluated by an attending dermatologist. In 84 cases, histopathological examination of skin biopsies were obtained, in many cases to distinguish between specific types of drug reaction, eg, DRESS syndrome versus exanthematous eruptions. The presence of eosinophils in dermal inflammatory infiltrates was considered to support a hypersensitivity reaction.
Each patient was informed of the nature of the study and signed a consent form approved by the Research Council and Ethics Committee of Hamedan University of Medical Sciences, Hamedan, Iran. Written informed consent was obtained from patients for publication of this study and for any accompanying images. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Exclusion criteria in this study included: 1) a history of taking more than one drug class likely to cause the adverse reaction; 2) clinical manifestations that were not compatible with drug reactions; 3) patient inability to produce the medication consumed the last 3 weeks that purportedly caused the reaction (to prevent recall bias); 4) cases involving overlapping diagnoses with other conditions; and 5) cases in which the clinical diagnosis did not match the findings cited in the pathology report.
Results
During the study period, 308 patients, including 114 men (37%) and 194 women (63%), were enrolled. Of the 308 patients, the diagnosis of ADR was made based on purely clinical manifestations in 224 cases; histological confirmation was obtained in 84 cases. The mean age of patients was 35.2±16.8 years (range 2–77 years). In the present study, ADRs were more frequently seen in the third and fourth decades of life, with 40% of reactions seen in this age group. The clinical manifestations of cutaneous ADRs are summarized in Table 1 and examples are provided in Figures 1 and 2. Only 20 of the 308 patients (6.5%) in our study reported a history of a previous drug reactions.
  | Table 1 Clinical manifestations of cutaneous adverse drug reactions |
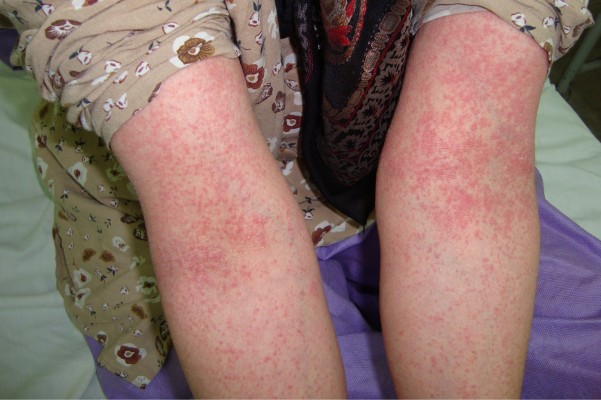
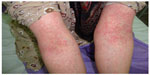  | Figure 1 A 25-year-old woman with the diagnosis of acute generalized exanthematous pustulosis following the use of cephalexin. |
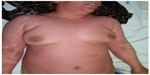  | Figure 2 A 37-year-old woman with clinical manifestation of DRESS syndrome due to phenytoin. |
In patients with urticarial drug eruptions, 76 (46.6%) were attributed to antibiotics, 45 (27.4%) to non-steroidal anti-inflammatory drugs (NSAIDs), and 17 (10.4%) to codeine with acetaminophen. In patients with maculopapular eruptions, 21 (45.6%) were attributed to antibiotic use, 12 (26%) to anticonvulsant drugs, and four (8.6%) to NSAIDs. In the beta-lactam and NSAID subgroups, amoxicillin and ibuprofen were the most common offending agents, respectively. In patients with fixed drug eruptions, 34 (59.6%) were attributed to taking trimethoprim-sulfamethoxazole and nine (15.7%) to taking NSAIDs (Figure 3).
In patients with a diagnosis of fixed drug eruption induced by trimethoprim-sulfamethoxazole (34 patients), most lesions were present in the genital area (18 patients). Five patients had lesions in both the genital area and the lip, and only one patient suffered from a lesion present only on the lip. The remaining patients had lesions in other areas, including the hands and feet. In the group taking NSAIDs (nine patients), one patient had a fixed drug eruption on the lip, one patient had it on the genital area, and two patients had lesions on both the lip and genital area. The remaining five patients had lesions on other body areas, including the hands, feet, and trunk.
Among the seven patients with erythema multiforme, two had history of taking trimethoprim-sulfamethoxazole. In this study, only one patient had a diagnosis of DRESS syndrome, which was attributed to phenytoin.
In this study, the most common cutaneous clinical manifestations of ADRs in the order of frequency were urticaria, maculopapular eruptions, and fixed drug reactions (Table 1). Antibiotics (42.7%) and NSAIDs (16.5%) were the most common causes of drug reactions in our study population.
Discussion
Consistent with the results of previous studies, we found that adverse cutaneous drug reactions are more common in women than in men.11 In the present study, 40% of drug reactions were seen in third and fourth decades of life, which is consistent with the findings of Sushma et al in their 2005 study.8
In the study performed by Souissi et al in 2007, the most common cutaneous clinical manifestations were maculopapular eruptions followed by fixed drug eruptions, and antibiotics and NSAIDs were the most commonly purported agents.12 Fiszenson-Albala et al, in a study of drug reactions in the French population, reported maculopapular eruptions followed by urticaria and erythroderma to be the most frequent ADRs in their population.13 Kacalak-Rzepka et al reported maculopapular eruptions and urticaria as the most common forms of ADR.11 The high prevalence of urticaria in our study may be due to the excessive use of beta-lactam antibiotics for the treatment of upper respiratory viral infections, often without proper medical indication.
In patients with a diagnosis of fixed drug eruption induced by trimethoprim-sulfamethoxazole, most lesions were seen in the genital area, whereas in the group taking NSAIDs, most lesions were seen on the hands and feet. In comparison, Justiniano et al reported that fixed drug eruptions induced by trimethoprim-sulfamethoxazole were most commonly present on the genital area, while those induced by NSAIDs were most commonly seen on the lip.14
It should be noted that our study does not address the mechanism of ADRs, and many drug reactions may not result from allergic or non-allergic drug hypersensitivity. In addition, a drug reaction cannot be confirmed without further testing, such as rechallenge, which was not performed in our study. Moreover, histologic examination may not reveal changes specific to a drug eruption, although biopsies may be helpful in distinguishing particular subtypes of reactions. Furthermore, a more helpful study may compare the adverse event rate to exposure rate by gathering local data on dispensing of various etiologic agents to estimate the cutaneous adverse event rate.
Conclusion
According to our results, adverse cutaneous drug reactions were mainly induced by beta-lactam antibiotics and NSAIDs. The most common forms of cutaneous ADRs in order of frequency were acute urticaria, fixed drug eruptions, and maculopapular rashes. In our study, the most common form of cutaneous ADR was found to be urticaria, while some studies conducted in other parts of the world have found maculopapular eruptions to be more common. The high consumption of beta-lactam antibiotics in the treatment of common viral upper respiratory infections in our area may contribute to our findings.
Disclosure
The authors have no conflicts of interest to declare.
References
Hunzicker T, Kazia P, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM):Adverse skin reaction a 20 year survey.Allergy. 1997;52(4):388–393. | |
Jean Revu Z, Allanore LV. Drug Reaction. In: Jean L Bolognia, Joseph L Jorizzo Julie, et al, editors.Dermatology. 3rd ed. Elsevier; 2012:335. | |
Charli-Joseph Y, Cruz-Fuentes C, Orozco-Topete R. Incidence of adverse cutaneous drug reaction in Mexican sample: an exploratory study on their association to tumour necrosis factor alpha TNF2 allele.J Eur Acad Dermatol Venereol. 2009;23(7):788–792. | |
Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions.Clin Dermatol. 2005;23(2):171–181. | |
Pichler WJ, Yawalkar N, Britschgi M, et al. Cellular and molecular pathophysiology of cutaneous drug reaction.Am J Clin Dermatol. 2002;3(4):299–238. | |
Hamilton RG, Adkinson NF Jr. Clinical laboratory assessment of IgE-dependent hypersensitivity.J Allergy Clin Immunol. 2003;111(2 Suppl):S687–S701. | |
Kimyai-Asadi A, Harris JC, Nousari HC. Critical overview: adverse cutaneous reactions to psychotropic medications.J Clin Psychiatry. 1999;60(10):714–725. | |
Sushma M, Noel MV, Ritika MC, James J, Guido S. Cutaneous adverse cutaneous drug: a 9 year study from a South Indian Hospital.Pharmacoepidemiology Drug Saf. 2005;14(8):567–570. | |
Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia JL, Lorizzo JL, Rapini RP, editors.Dermatology, 2nd ed. Spain: Mosby-Elsevier; 2008:301–320. | |
Roujean JC. Epidemiology of drug reaction, service de dermatologie, Universite Paris. 2004;13:322–328. | |
Kacalak-Rzepka A, Klimowicz A, Bielecka-Grzela S, Załuga E, Maleszka R, Fabiańczyk H. Retrospective analysis of adverse cutaneous drug reactions in patients.Ann Acad Med Stetin. 2008;54(2):52–58. | |
Souissi A, Fenniche S, Benmously R, Ben JS, Marrak H, Mokhtar I. Study of the cutaneous drugs reactions in a teaching hospital in Tunis.Tunis Med. 2007;85(12):1011–1015. | |
Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6 month prospective survey of cutaneous drug reaction.Br J Dermatol. 2003;149(5):1018–1022. | |
Justiniano H, Berlingeri-Ramos AC, Sanchez JL. Pattern analysis of drug-induced skin disease.Am J Dermatopathol. 2008;30(4):352–369. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.