Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration
Authors Tichota D, Silva AC , Sousa Lobo JM, Amaral MH
Received 13 March 2014
Accepted for publication 27 April 2014
Published 11 August 2014 Volume 2014:9(1) Pages 3855—3864
DOI https://doi.org/10.2147/IJN.S64008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Deise Michele Tichota,1 Ana Catarina Silva,2,3 José Manuel Sousa Lobo,2 Maria Helena Amaral2
1Federal University of Rio Grande do Sul, Porto Alegre, 2Laboratory of Pharmaceutical Technology/Centre of Research in Pharmaceutical Sciences, Faculty of Pharmacy, University of Porto, 3Faculty of Health Sciences, Fernando Pessoa University, Porto, Portugal
Abstract: Given its advantages in skin application (eg, hydration, antiaging, and protection), argan oil could be used in both dermatological and cosmetic formulations. Therefore, the preparation of nanostructured lipid carriers (NLCs) using argan oil as a liquid lipid is a promising technique, since the former constitute well-established systems for dermal delivery. The aim of this work was to develop a topical formulation of argan oil NLCs to improve skin hydration. Firstly an NLC dispersion was developed and characterized, and afterward an NLC-based hydrogel was prepared. The in vivo evaluation of the suitability of the prepared formulation for the proposed application was assessed in volunteers, by measuring different skin-surface parameters for 1 month. An argan oil NLC-based hydrogel formulation was successfully prepared and characterized. Moreover, the entrapment of the NLCs in the hydrogel net did not affect their colloidal sizes. Additionally, it was observed that this formulation precipitated an increase in skin hydration of healthy volunteers. Therefore, we concluded that the preparation of NLC systems using argan oil as the liquid lipid is a promising strategy, since a synergistic effect on the skin hydration was obtained (ie, NLC occlusion plus argan oil hydration).
Keywords: argan oil, nanostructured lipid carriers, NLC, hydrogels, skin hydration
Introduction
The skin is the major and outermost organ of the body, and performs several important physiological functions. This structure is formed by two layers: the epidermis and dermis. The former is more external and ends with the stratum corneum (SC), which plays an important barrier function, protecting the body inside from the external environment.1,2
The SC surface displays a hydrolipidic film composed of water, hygroscopic compounds (natural moisturizing factors), and lipid compounds that produce an occlusive effect. Both natural moisturizing factors and lipids form a barrier that has the ability to prevent water loss by evaporation, helping to maintain normal skin water content. The normal functioning of the SC can be disturbed under dry-skin conditions. When this occurs, the effectiveness of the SC-barrier function stops and a cycle of events initiates, such as superficial dehydration of the SC, subsequent release of inflammatory mediators, induction of epidermal keratinocyte hyperproliferation, and disruption of epidermal cellular differentiation.1 Accordingly, the evaluation of skin hydration has gained a growing interest in recent years, particularly in the field of experimental dermatology. Several in vivo and in vitro methods have been proposed for the determination of skin hydration. Nonetheless, in vivo methods provide more realistic information. Among these, electrometric techniques have been the most applied. These are based on the determination of electrical changes (impedance, resistance, and capacitance) that are detectable at the skin surface, by applying different electrical currents. The skin parameters evaluated more often are SC hydration, sebum content, microrelief, and transepidermal water loss (TEWL).3 The measurement of SC hydration gives information about the amount of water present in this layer. The sebum is composed of a lipid mixture produced by sebaceous glands, which has an important role in the maintenance of the SC-barrier function.4 Skin microrelief is used to evaluate skin-hydration efficacy or the antiaging effects of cosmetics, and could be assessed by measuring the parameters of roughness, scaling, smoothing, and wrinkling.5,6 TEWL is indicative of dehydration processes occurring, which could compromise the effectiveness of the SC-barrier function.7 The use of moisturizers influences the skin-barrier function by reducing TEWL. Moreover, this influence depends on the composition of the moisturizer.8 An efficient moisturizer formulation reduces dry skin and irritation, avoiding the conditions that can lead to skin disease.9
Nowadays, nanostructured lipid carriers (NLCs) are well-established systems that have been successfully used for dermal delivery of cosmetics and drugs. These carrier systems consist of aqueous dispersions of solid nanoparticles, composed of a mixture of solid and liquid lipids, and stabilized by one or two surfactants. The excipients used in NLC systems are generally recognized as safe substances, which predicts an absence of toxicity for topical application. Moreover, NLCs have been described as efficient systems to improve skin hydration, due to their physiological lipid composition and occlusive effect properties. Typically, NLC dispersions present a low viscosity, which is not advantageous for topical application, because it decreases the time of permanence at the application site. To avoid this, NLCs can be incorporated into traditional semisolid systems (eg, hydrogels [HGs]), increasing the consistency of final formulations and also the long-term stability of the incorporated nanoparticles.10,11
Argan oil is a natural oil that has been applied in cosmetics, because of its antioxidant, hydration, antiaging, and protection properties on the skin.12 Based on the aforementioned properties, the preparation of NLC systems using argan oil as the liquid lipid is a promising technique. Therefore, the aim of this work was to develop a topical formulation of argan oil NLC to improve skin hydration. For this, firstly an NLC dispersion was developed and characterized, and afterward an NLC-based HG was prepared (HG-NLC). The in vivo evaluation of the suitability of the prepared formulation for the proposed application was assessed in volunteers by measuring different skin-surface parameters for 1 month.
Materials and methods
Materials
Argan oil, the gelling agent PFC® (carbomer 2001) and triethanolamine were purchased from Acofarma (Madrid, Spain). Precirol® ATO5 (glyceryl palmitostearate) and Apifil® (polyethylene glycol-8 beeswax) were kindly provided by Gattefossé (Saint-Priest, France). Witepsol® E 85 (hydrogenated cocoglycerides), Dynasan® 114 (glyceryl trimyristate) and Softisan® 142 (hydrogenated cocoglycerides) were gifts from Sasol (Witten, Germany). Cetrimide and Tween® 80 (polysorbate 80) were obtained from JM Vaz Pereira (Sintra, Portugal) and Guinama (Valencia, Spain), respectively. Purified water (Milli-Q® Plus) was obtained from EMD Millipore (Billerica, MA, USA).
Screening of lipid excipients
When developing an NLC dispersion, the choice of the most suitable solid–liquid lipid combination, which leads to the formation of an appropriate solid nanoparticle matrix, is fundamental.13,14 Accordingly, the miscibility of argan oil with five different solid lipids (Apifil, Dynasan 114, Precirol ATO5, Softisan 142 and Witepsol E85) was evaluated in increasing proportions, ranging from 50:50 to 90:10 (solid lipid:liquid lipid). For this, various physical mixtures of lipids were heated until 100°C±1°C for 1 hour, with stirring (200 rpm). Afterward, the mixtures were cooled until room temperature (20°C±1°C) for solidification. The existence/absence of miscibility between the two lipids was analyzed by placing a portion of each solidified mixture on a filter paper, followed by visual observation, to verify the presence of oil drops, which would be indicative of a lack of miscibility between lipids.15
Preparation of NLC dispersions
The NLC dispersions were prepared according to the method previously employed by Silva et al.16 Briefly, the lipids were heated at 5°C–10°C above the melting point of the solid lipid (56°C). At the same time, the aqueous phase, composed of surfactant, preservative, and purified water, was heated at the same temperature. After melting of the lipid phase, the aqueous phase was added to the former and homogenized under high-speed stirring, using an Ultra-Turrax® T25 (IKA, Staufen, Germany), at 8,000 rpm for 5 minutes. The pre-emulsion obtained was placed under a probe sonicator (Sonic & Materials, Newtown, CT, USA), with a power-output amplitude of 70% for 15 minutes, to allow for oil-droplet breakdown. Subsequently, the hot nanoemulsion formed was transferred to glass vials and cooled to room temperature to form the solid nanoparticles, ie, the NLCs.
Preparation of hydrogels
For the preparation of the HG-NLC, the gelling agent PFC was first powdered in a porcelain mortar. Afterward, the NLC dispersion was added to the mortar and the polymer was neutralized to pH 7 with triethanolamine, to allow for the formation of the HG. A control HG without NLCs was also prepared to compare the results of all performed studies.
Particle-size, polydispersity-index, and zeta-potential measurements
The diameter (Z-ave) and particle-size distribution (evaluated by the polydispersity index [PI]) are parameters that have a direct impact on the physical stability of a dispersion. Photon correlation spectroscopy, also known as dynamic light scattering (DLS), and laser diffractometry (LD) are the most widely used techniques for measuring the size and distribution of particles in colloidal dispersions.17 Furthermore, during the optimization of an NLC dispersion, it is important to verify the presence/absence of microparticles that are not detectable by common DLS equipment.16 Accordingly, the particle-size measurement by LD using a Mastersizer 2000E (Malvern Instruments, Malvern, UK) was first performed. For this purpose, volume distribution of 10%, 50%, and 90% was measured, which referred to particles with diameters equal or lower than the given values. Afterward, to confirm the presence of particles in the colloidal size range, the Z-ave and PI of the prepared NLC dispersions were measured on the production day with DLS (ZetaPALS; Brookhaven Instruments, Holtsville, NY, USA). Before the measurements, the dispersions were diluted (1:200) with ultrapure water, to avoid the light multiscattering related to a high concentration of particles.
The zeta potential (ZP) refers to the total surface charge that a particle acquires in a given environment, and can be indicative of good long-term stability of the dispersions.17,18 ZP measurements were performed using the ZetaPALS apparatus. For this, the dispersions were previously diluted with ultrapure water to a suitable concentration. All the DLS and ZP experiments were performed at room temperature (20°C±1°C), and the results presented are average values of six measurements (n=6) plus standard deviation (SD).
The mean particle size of the NLCs was also evaluated after the preparation of the HG, in order to verify if the particles maintained their nanometric sizes after integration within the HG structure. The network of the HG was previously destroyed by dilution with purified water and vortexing, and Z-ave, ZP, and PI were evaluated as described previously for the NLC dispersions alone.
In order to estimate the long-term stability of all the prepared systems, the size of the nanoparticles was analyzed after storage at room temperature (20°C±1°C) and in the refrigerator (5°C±1°C) for 90 days.
Cryo-scanning electron microscopy
The structure of the NLC dispersions alone and after incorporation in the HG was observed by cryo-scanning electron microscopy (cryoSEM). For this, the samples were mounted on metal stubs, rapidly frozen with slush nitrogen until −210°C, sublimated at −90°C for 90 seconds, and coated with a mixture of gold and palladium under vacuum. Subsequently, the samples were fractured, transferred to the chamber, and examined using SEM (JSM-6301F; JEOL, Tokyo, Japan)/INCA Energy 350 (Oxford Instruments, Abingdon, UK)/ALTO 2500 (Gatan, Pleasanton, CA, USA). The observations were done at −150°C.
pH analysis
The determination of the pH of a formulation intended for cutaneous application is extremely important, since it must be compatible with the pH of the application site. The natural pH of the skin comes from the secretions of sweat and sebaceous glands, and lactic acid production, which leads to the formation of a protective film over the entire skin surface, designated hydrolipidic film. The skin normally has an average pH of 5.5, although this may vary slightly depending on the area of the body.19
The evaluation of the pH was performed in all prepared HGs on days 7 and 30 after storage at different temperatures. For this, a glass pH electrode (Basic 20; Crison Instruments, Barcelona, Spain) was directly dipped in each semisolid formulation. All analyses were performed in triplicate (means ± SD).
Texture analysis
The evaluation of texture parameters, such as adhesiveness and firmness, of semisolid formulations provides information about their mechanical properties. Texture analyses were performed using a probe that dips in the formulation, with a defined velocity and force. The results of force versus distance were plotted, allowing the calculation of adhesiveness (negative area) and firmness (maximum force) of the formulation. In more detail, adhesiveness is related with bioadhesion and is a measure of the force required to overcome the attractive forces between the surfaces of the sample and the probe. On the other hand, firmness is related to the ease of product application on the skin.20–22 A texture analyzer (TA-XT2i; Stable Micro Systems, Godalming, UK) was used to carry out the texture analysis. The compression mode was applied to perform a penetration test using a load cell of 5 kg, a trigger force of 0.05 N, a cylindrical probe (25 mm diameter), a penetration depth of 5 mm, and a test speed of 3 mm·s−1. All the experiments were done in triplicate (means ± SD), at room temperature (20°C±1°C), on days 7 and 30, after the preparation of the HGs, in order to study the effects of NLC incorporation and storage conditions on their textural properties.
Rheological measurements
According to the increase effect on the formulation consistency, the use of lipid-nanoparticle dispersions by means of semisolid formulations has been presented as a good alternative to improve their topical application.11,23–25 The study of the flow properties (ie, consistency) of semisolid formulations gives information about their rheological behavior, which allows estimation of their suitability for the proposed application. These studies can be performed in viscometers, and the respective flow behavior can be assessed by analysis of the plots of shear stress versus shear rate.22,26,27 Nonetheless, the use of mathematical models that fit the rheological data is important to confirm the flow behavior graphically observed. Therefore, two models were applied:28,29 power law (Ostwald–de Waele), and power law with a yield stress (Herschel–Bulkley). The flow index value (n) was used to assess the rheological behavior of the formulations.
Rheological tests were performed on a rotational viscometer (Haake Viscotester™ 550; Thermo Fisher Scientific, Waltham, MA, USA), with an SV/DIN coaxial cylinder sensor. The flow behavior of the developed HG was studied as in our previous work,22 by continuous shear investigations, which were performed in order to evaluate the shear stress (Pa) as a function of shear rate (s−1). The study started with a shear rate of 1.0·s−1, went up to a maximum of 500·s−1, and then back to 1.0·s−1, and the resulting shear stress was measured. To reduce the influence of temperature on the rheological behavior of the HG, a thermostatic water bath was used to accurately maintain the sample temperature (20°C±1°C) during all experiments. The rheological measurements were performed on days 1 and 30, in order to assess the effects of NLC incorporation and storage conditions on the flow properties of the HGs.
Color measurements
The detection of changes in the color of the semisolid formulations can indicate the occurrence of degradation on their components. In addition, it is well known that lipids may undergo degradation resulting from oxidation reactions that may occur during thermal processing and/or storage. Typically, lipid-oxidation reactions generate colored compounds. Therefore, the assessment of the occurrence of color changes in the prepared formulations can give information about lipid stability.30,31
Color determination based on the color space L*a*b* was performed using a colorimeter (Chroma Meter CR-500; Konica Minolta, Tokyo, Japan), with a D65 light source and an observation angle of 2°. The L* means the amount of reflected light, and can range from 0 (black) to 100% (white); the a* and b* represent, respectively, the colors from green to red or blue to yellow, and the values range from –60 (close to green or blue) to +60 (close to red or yellow). Through the values of a* and b*, the chrome parameter (C*) can be calculated, which reveals the color of the formulation and allows the detection of color changes. C* can be obtained by applying the equation 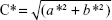 .32,33 The parameter C* was evaluated in both HG and HG-NLC formulations, on the production day, and after 30 days of storage at different temperatures. All determinations were performed in triplicate, and the results were calculated by mean values (n=3± SD).
.32,33 The parameter C* was evaluated in both HG and HG-NLC formulations, on the production day, and after 30 days of storage at different temperatures. All determinations were performed in triplicate, and the results were calculated by mean values (n=3± SD).
Human in vivo evaluation of skin hydration
Design of the study
The study was planned as a single-blinded, controlled trial, and was performed for 1 month in ten healthy-skin Caucasian volunteers of both sexes aged between 21 and 30 years. Prior to the experiments, all participants were informed about the methodologies of testing, and signed the informed consent. The latter was prepared according to the recommendations of the Declaration of Helsinki, which state the ethical principles that should be accomplished for research involving human subjects.34 Before the experiments, the ten volunteers were randomly divided in two groups. Group 1 consisted of five volunteers who applied the HG-NLC formulation. Group 2 consisted of five volunteers who applied the HG formulation. The exclusion criterion was the existence of skin damage on both forearms of the volunteers. On the first day of the study, volunteers could not apply any product on both forearms. Prior to the measurements, the volunteers were made to rest for at least 30 minutes in a room with both controlled temperature (20°C±1°C) and relative humidity (45%±1%). The procedures were determination of the application site, with a distance of 5 cm above the wrist and 5 cm below the elbow, and measurement of skin hydration, sebum, TEWL, scaliness, smoothness, roughness, and energy parameters. Volunteers were instructed to apply the formulation once a day for 1 month. The forearm with no formulation application was used as a control. All the measurements were performed in triplicate (means ± SD) on day 0 and 30.
Assessment of skin hydration
For the assessment of skin hydration, a Multi Probe Adapter System was used (Courage + Khazaka Electronics, Cologne, Germany), which consists of a basic device connected to different probes that allows for the determination of different skin parameters: surface hydration (Corneometer® CM 825), and lipid content (Sebumeter® SM 810). For microrelief analysis the Visioscan® VC 98 (Courage + Khazaka Electronics), was used to evaluate the following quantitative topographic characteristics: scaliness, smoothness, roughness, and energy. The skin-barrier function was evaluated by TEWL measurement using the Tewameter® TM 210 (Courage + Khazaka Electronics).
Statistical analysis
Statistical analysis of the results of skin-hydration evaluations was performed applying one-way analysis of variance (α=0.05) and Tukey posttests, using SPSS version 21.0 (IBM, Armonk, NY, USA). A P-value <0.05 was considered statistically significant.
Results and discussion
Screening of lipid excipients
The results of the miscibility tests demonstrated that both Precirol ATO5 and Softisan 142 were suitable for the preparation of lipid nanoparticles containing argan oil. It was found that these solid lipids exhibited a miscibility with argan oil at a proportion of 70:30 (solid lipid:argan oil), while the other lipids tested were miscible only at the proportions of 80:20 and 90:10 (data not shown). Therefore, we selected these two lipids to prepare NLC dispersions (Table 1).
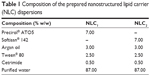  | Table 1 Composition of the prepared nanostructured lipid carrier (NLC) dispersions |
Particle-size, polydispersity-index, and zeta-potential measurements
According to the LD measurements, both NLC dispersions (with Precirol ATO5 or Softisan 142) revealed the presence of 90% of particles with sizes in the nanometer range (data not shown). Nonetheless, the NLC1 dispersion had smaller and more homogeneous nanoparticles. Therefore, we selected Precirol ATO5 to prepare the NLCs used for the subsequent studies.
Table 2 presents the results of Z-ave, PI, and ZP of the NLC1 dispersion, measured on the production day and after storage at different temperatures. From Table 2, we can see that all dispersions revealed the presence of nanosized particles (100–200 nm), PI values lower than 0.300, and ZP higher than |30| mV, which have been described as acceptable for topical delivery. Moreover, after storage, no significant variations on these values were observed, indicating good long-term stability of dispersions.10,35
The composition of the prepared HGs (HG-NLC and HG) is shown in Table 3. Table 4 presents the NLC Z-ave after the preparation of the HG. From Table 4, we can observe that regarding Z-ave and PI values, no relevant alterations occurred. Therefore, we concluded that the NLC dispersions remained stable after incorporation in the HG network. Moreover, stability was observed during storage at different temperatures. Similar results have been described elsewhere.22,36 In contrast, a high variation in ZP values was observed, which could have been related to the negative charge of the carboxylic groups present on the gelling agent (PFC). Nonetheless, the values remain acceptable, since they are, in absolute value or modulus, higher than 30 mV.35
  | Table 3 Composition of the prepared hydrogels (nanostructured lipid carrier-based hydrogel [HG-NLC] and control hydrogel [HG]) |
Cryo-scanning electron microscopy
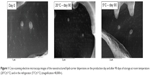
Figure 1 presents the typical structure of lipid nanoparticles, with almost spherical shape and smooth surface.27,37 After 90 days of storage at different temperatures, no visible changes on the NLC aspect were observed, which is in agreement with the results obtained for Z-ave, PI, and ZP.
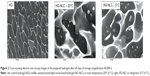
From Figure 2, we can visualize a typical HG network (left)38 and the presence of NLC within the HG net (middle and right) after storage at different temperatures. From the latter, we conclude that the HG formulation is effective for the incorporation of NLCs without changing their stability, which is in accordance with the results obtained for Z-ave. Similar images of lipid-based HG systems were presented by Silva et al.22
pH analysis
The results obtained reveal pH values between 7 and 8 (data not shown), which are acceptable for cutaneous application.39 Moreover, it was observed that the temperature and storage time did not influence these values.
Texture analysis
The results of texture analysis are shown in Figure 3. From Figure 3A, we can observe that the presence of NLCs precipitated a high decrease in the firmness of the HG. The maximum force (ie, firmness) of the HG exhibited values between 0.7 and 0.8 N, while the maximum force of the HG-NLC ranged between 0.2 and 0.3 N. In addition, no significant changes in the firmness of any formulations were observed after storage at different temperatures over 30 days.
With regard to adhesiveness (Figure 3B), the presence of NLCs also caused a decrease in the values of HG-NLC, when compared to the control (HG). The latter had adhesiveness values between −1.5 and −2.5 N·mm. In contrast, for the former, the values ranged between −1.0 and −1.5 N·mm. Additionally, there were no significant changes in these values over the 30 days of storage at different temperatures.
Typically, an adequate topical semisolid formulation should present major mechanical properties, namely high adhesiveness to prolong contact time, and good firmness to facilitate its local application.40 The decrease in firmness and adhesiveness observed for the HG-NLC, could have been related to the disruption of the HG structure, originated by the presence of the NLC dispersion. This phenomenon could also be observed in cryoSEM images (Figure 2). Similar results were described by Gonzalez-Mira et al.36
Rheological measurements
Rheological measurements were performed on the HG-NLC formulation and compared with the HG in order to study the effect of the presence of lipid nanoparticles on the flow behavior of the semisolid formulation. In addition, the effect of storage at different temperatures was evaluated (Figure 4, A and B).
From the analysis of the rheograms (Figure 4, A and B), it can be seen that the formulations showed a non-Newtonian behavior. These HGs exhibited a decrease in apparent viscosity with an increasing shear rate, ie, a plastic or a pseudoplastic with yield-value behavior. Furthermore, no thixotropy was observed, because there was no decrease in the apparent viscosity of the formulations with time (the upper and lower curves of the rheograms overlap).26 These data corroborate the results found after the application of the rheological models, where the n-value was less than 1, which indicates a shear thinning behavior.28,29
From the observation of all rheograms, it can be seen that the HG-NLC showed lower viscosity than the HG, indicating that the presence of lipid nanoparticles influences the consistency of the HG, leading to a decrease in viscosity. These results are in agreement with those obtained in the texture analysis. Furthermore, it was observed that during storage, all formulations underwent a slight decrease in viscosity.
Color analysis
The values obtained for the chrome parameter (C*) showed that after 30 days of storage, only minor changes were detected for the HG-NLC, while the HG maintained its initial values (data not shown). Despite the former formulation not exhibiting color changes visible to the naked eye, a slight decrease in the C* values was detected at both storage temperatures (data not shown). Nonetheless, these variations were considered statistically irrelevant. Accordingly, it was concluded that there were no changes in the color of the formulation during storage, which could indicate the nonoccurrence of lipid degradation (by means of oxidation reactions), and reveals lipid stability during thermal process and storage.31 It should be noted that the presence of argan oil could have contributed to this effect, according to its described antioxidant properties.41
Human in vivo evaluation of skin hydration
The most relevant results of the human in vivo evaluation of skin-hydration tests are presented in Figure 5. From the results, it can be seen that after application of the HG-NLC formulation, for 30 days, there was a significant increase in the skin hydration (P<0.05) when compared to the HG (blank) application. However, the differences between the hydration of the forearm where was applied the HG-NLC formulation, and the forearm control where not statistically significant (P>0.05) (data not shown). Besides, for the HG, the differences were not statistically significant (P>0.05). Additionally, in the data (not shown) obtained for the other evaluated skin parameters (lipid content, TEWL, scaliness, smoothness, roughness, and energy), there were no statistically significant differences between the values (P>0.05) obtained before and after 30 days of HG-NLC application.
The significant increase in skin hydration observed for the HG-NLC formulation could be attributed to the presence of lipid nanoparticles. The significant increase in skin hydration observed for the HG-NLC formulation could be attributed to the presence of lipid nanoparticles, which have displayed a skin-moisturizing effect when incorporated in semisolid formulations.42–44 Besides, this effect could also be attributed to the argan oil, which has been described as a compound with skin-moisturizing properties.12
Conclusion
An argan oil NLC-based HG formulation was successfully prepared and characterized. Moreover, the entrapment of the NLCs in the HG net did not affect their colloidal sizes. Additionally, it was observed that this formulation resulted in an increase in the skin hydration of healthy volunteers.
According to the results obtained in this study, we conclude that the preparation of NLC systems using argan oil as the liquid lipid is a promising technique, since they could have a synergistic effect on skin hydration (ie, NLC occlusion plus argan oil hydration). Moreover, the solubilization of an additional cosmetic active ingredient (eg, an antiaging compound) in this oil would improve interest in the NLC system for skin application.
Acknowledgments
This work was supported by both the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) of Brazil. The authors are thankful to Paralab for providing the Mastersizer for particle-size analysis, and also to Professor Sallete Reis, from the Department of Chemistry and Physics, Faculty of Pharmacy, Porto University, for the use of the ZetaPALS apparatus for particle-size analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005;124(6):1099–1110. | ||
Menon GK. New insights into skin structure: scratching the surface. Adv Drug Deliv Rev. 2002;54 Suppl 1:S3–S17. | ||
Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980;75(6):500–507. | ||
Darlenski R, Sassning S, Tsankov N, Fluhr JW. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009;72(2):295–303. | ||
Fischer TW, Wigger-Alberti W, Elsner P. Direct and non-direct measurement techniques for analysis of skin surface topography. Skin Pharmacol Physiol. 1999;12(1–2):1–11. | ||
Lagarde JM, Rouvrais C, Black D, Diridollou S, Gall Y. Skin topography measurement by interference fringe projection: a technical validation. Skin Res Technol. 2001;7(2):112–121. | ||
Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. | ||
Buraczewska I, Berne B, Lindberg M, Törmä H, Lodén M. Changes in skin barrier function following long-term treatment with moisturizers, a randomized controlled trial. Br J Dermatol. 2007;156(3):492–498. | ||
Simion FA, Abrutyn ES, Draelos ZD. Ability of moisturizers to reduce dry skin and irritation and to prevent their return. J Cosmet Sci. 2005;56(6):427–444. | ||
Müller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN & NLC): present state of development and industrial applications. Curr Drug Discov Technol. 2011;8(3):207–227. | ||
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–184. | ||
Guillaume D, Charrouf Z. Argan oil and other argan products: use in dermocosmetology. Eur J Lipid Sci Technol. 2011;113(4): 403–408. | ||
Chakraborty S, Shukla D, Mishra B, Singh S. Lipid – an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73(1):1–15. | ||
Mendes AI, Silva AC, Catita JA, Cerqueira F, Gabriel C, Lopes CM. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf B Biointerfaces. 2013;111C:755–763. | ||
Hommoss A. Nanostructured Lipid Carriers (NLC) in Fermal and Personal Care Formulations [doctoral thesis]. Nerlin: Freie Universität Berlin; 2008. | ||
Silva A, González-Mira E, García M, et al. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf B Biointerfaces. 2011;86(1):158–165. | ||
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2):165–196. | ||
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–469. | ||
Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6): 296–302. | ||
Jones D, Woolfson AD, Brown A. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm Res. 1997;14(4): 450–457. | ||
Jones DS, Lawlor MS, Woolfson AD. Examination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-co-maleic anhydride) and poly(vinylpyrrolidone): rheological and mathematical interpretation of textural parameters. J Pharm Sci. 2002;91(9):2090–2101. | ||
Silva AC, Amaral MH, González-Mira E, Santos D, Ferreira D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: preparation and characterization studies. Colloids Surf B Biointerfaces. 2012;93:241–248. | ||
Lippacher A, Müller RH, Mäder K. Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles. Int J Pharm. 2001;214(1–2):9–12. | ||
Jeon HS, Seo JE, Kim MS, et al. A retinyl palmitate-loaded solid lipid nanoparticle system: effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int J Pharm. 2013;452(1–2):311–320. | ||
Puglia C, Damiani E, Offerta A, et al. Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: characterization, in vitro penetration and photostability studies. Eur J Pharm Sci. 2014;51:211–217. | ||
Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release. 2009;136(2):88–98. | ||
Silva AC, Santos D, Ferreira DC, Souto EB. Minoxidil-loaded nanostructured lipid carriers (NLC): characterization and rheological behaviour of topical formulations. Pharmazie. 2009;64(3): 177–182. | ||
Moreno R. Rheology. In: Buschow KH, Cahn RW, Flemings MC, et al, editors. Encyclopedia of Materials: Science and Technology. Oxford: Elsevier; 2001:8192–8196. | ||
Marcotte M, Taherian Hoshahili AR, Ramaswamy HS. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res Int. 2001;34(8):695–703. | ||
Gray JI. Measurement of lipid oxidation: a review. J Am Oil Chem Soc. 1978;55(6):539–546. | ||
Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):734–746. | ||
Tárrega A, Costell E. Colour and consistency of semi-solid dairy desserts: instrumental and sensory measurements. J Food Eng. 2007;78(2): 655–661. | ||
Sagawa K. Toward a CIE supplementary system of photometry: brightness at any level including mesopic vision. Ophthalmic Physiol Opt. 2006;26(3):240–245. | ||
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. | ||
Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. | ||
Gonzalez-Mira E, Nikolić S, Calpena AC, Egea MA, Souto EB, García ML. Improved and safe transcorneal delivery of flurbiprofen by NLC and NLC-based hydrogels. J Pharm Sci. 2012;101(2):707–725. | ||
Hatziantoniou S, Deli G, Nikas Y, Demetzos C, Papaioannou GT. Scanning electron microscopy study on nanoemulsions and solid lipid nanoparticles containing high amounts of ceramides. Micron. 2007;38(8):819–823. | ||
Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. | ||
Stefaniak AB, Plessis JD, John SM, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: part 1. pH. Skin Res Technol. 2013;19(2):59–68. | ||
Jones DS. Dynamic mechanical analysis of polymeric systems of pharmaceutical and biomedical significance. Int J Pharm. 1999; 179(2):167–178. | ||
Charrouf Z, Guillaume D. Argan oil: occurrence, composition and impact on human health. Eur J Lipid Sci Technol. 2008;110(7):632–636. | ||
Wissing SA, Müller RH. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity – in vivo study. Eur J Pharm Biopharm. 2003;56(1):67–72. | ||
Pardeike J, Schwabe K, Müller RH. Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect. Int J Pharm. 2010; 396(1–2):166–173. | ||
Loo C, Basri M, Ismail R, et al. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int J Nanomed. 2013;8:13–22. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.