Back to Journals » Nutrition and Dietary Supplements » Volume 6
Dermaval™ inhibits glucose-induced neutrophil elastase activity in healthy subjects
Authors Reyes-Izquierdo T, Nemzer B, Argumedo R, Shu C, Pietrzkowski Z
Received 31 August 2013
Accepted for publication 29 October 2013
Published 23 December 2013 Volume 2014:6 Pages 1—7
DOI https://doi.org/10.2147/NDS.S53838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Tania Reyes-Izquierdo,1 Boris Nemzer,2 Ruby Argumedo,1 Cynthia Shu,1 Zb Pietrzkowski1
1Applied BioClinical Inc., Irvine, CA, 2FutureCeuticals Inc, Momence, IL, USA
Background: Dermaval™ is a composite formulation of various phytochemical-rich plant materials as quantified using high-performance liquid chromatography. This blend exerts inhibitory activity on human neutrophil elastase (HNE). An acute, crossover clinical study was performed to assess the effects of Dermaval on glucose-induced HNE activity in 20 healthy subjects.
Methods: Participants served as their own controls during this 3-day trial. On day 1, all study participants were fasted and given only 300 mL of water. Blood was drawn before treatment and 60 and 120 minutes post treatment. On day 2, participants were fasted, treated with 75 g of glucose, and similarly tested. On day 3, participants consumed a 50 mg serving of Dermaval followed by 75 g of glucose 15 minutes later. HNE concentration and HNE total activity were determined using enzyme-linked immunosorbent assay.
Results: Average values for HNE activity in the control group (day 1) did not change. Treatment with a single dose of glucose (day 2) increased blood HNE activity by 175% over baseline levels (P=0.005) during the first 60 minutes. Pretreatment with Dermaval on day 3 prevented the glucose-induced increase in HNE activity (P=0.005 at 60 minutes and P=0.03 at 120 minutes versus respective day 2 values). Interestingly, ingestion of 75 g of glucose resulted in the same blood glucose levels on days 2 and 3, indicating that ingestion of Dermaval did not affect glucose absorption.
Conclusion: These results suggest that Dermaval acutely inhibits glucose-induced HNE activity. Further investigations are needed to elucidate the direct and/or indirect mechanism of this effect and to verify whether treatment with Dermaval without glucose may acutely affect activity of HNE as well.
Keywords: crossover study, human neutrophil elastase, glucose
Introduction
Elastases form a subfamily of serine proteases that hydrolyze many proteins in addition to elastin. In humans, there are six elastase genes that encode the structurally similar proteins, elastase 1, 2, 2A, 2B, 3A, and 3B. Elastase 2, ie, human neutrophil elastase [HNE] (EC 3.4.21.37) also known as lysosomal elastase, polymorphonuclear leukocyte elastase, elastase, serine elastase, lysosomal elastase, granulocyte elastase,1 and leukocyte elastase,2–4 hydrolyzes proteins within specialized neutrophil lysosomes and in the extracellular matrix. HNE plays a role in degenerative and inflammatory diseases by proteolysis of collagen IV and elastin in the extracellular matrix. For example, Schmelzer et al5 reported that HNE plays an important role in the degradation of skin elastin in older individuals. In vitro studies have demonstrated that ?broblasts cultured in three-dimensional collagen gels are able to contract these gels and that HNE can enhance the contractile process.6 HNE contributes significantly to ultraviolet-induced elastin damage, which is a critical part of ultraviolet-accelerated aging.7 Interestingly, fibroblast elastase produced by dermal fibroblasts was also found to play a crucial role in the degradation of skin elastin induced by ultraviolet radiation.8,9
These and similar observations have prompted many groups to search for inhibitors of HNE. Such inhibitors might be useful to reduce inflammation and to modulate the process of skin aging and formation of wrinkles.10 Several phytochemicals and chemicals are reported to inhibit HNE under various experimental conditions. Hydoroxyhibiscone A, for example, is reported to inhibit HNE activity in vitro.11 Components of specific essential oils are also reported to have anti-HNE activity.12 Ellagic and tannic acids inhibit premature degradation of elastin fibers in vitro,13 as do quercetin14 and punicalagin.15 However, the literature indicates scant evidence of the ability of these and similar phytochemicals to inhibit HNE activity in vivo.
HNE is a potent protease that can degrade most extracellular matrix components and other host proteins, such as clotting factors, complement, and immunoglobulins. The enzyme contributes to a proinflammatory process.16,17 HNE activity is under tight control by endogenous inhibitors, such as alpha 1-antitrypsin,18 secretory leukocyte protease inhibitor,19,20 monocyte/neutrophil elastase inhibitor,21,22 and pre-elafin (also known as skin-derived antileukoprotease, trappin-2, or WAP-3).23 As reported by Esposito et al,24 acute hyperglycemia results in an acute proinflammatory reaction characterized by increased levels of cytokines in the blood. Hyperglycemia also causes an acute increase in neutrophils, a major source of neutrophil elastase.25,26 Leukocyte elastase levels also correlate with circulating levels of blood glucose.27 Therefore, we hypothesized that transient hyperglycemia in otherwise healthy volunteers would induce HNE activity in serum.
Dermaval™ (VDF FutureCeuticals, Inc., Momence, IL USA) is a novel proprietary blend of various plant materials rationally designed to include several phytochemicals, including quercetin, punicalagin, and chlorogenic and neochlorogenic acids, in an amount and ratio necessary to inhibit the enzymatic activity of HNE in blood. The current study was designed to measure the acute effect of a single serving of Dermaval on blood levels of HNE specific enzymatic activity in healthy human subjects. Given that standard values for total serum HNE and HNE activity levels are not yet established for humans and hyperglycemia is known to increase proinflammatory cytokine levels,24,28 we used a single dose of glucose to increase and generally standardize HNE activity in the blood of young healthy study subjects.
Materials and methods
Clinical study parameters
This study was conducted according to the guidelines set out in the Declaration of Helsinki and all procedures involving human subjects were approved by the institutional review board at Vita Clinical SA (study protocol ABC-13-05-DML-1). All study subjects received a consent form in which they completed health-related questions prior to selection. All subjects were healthy and had not been using any type of medication or supplement for a period of 15 days prior to the start of the study. The inclusion criteria required participants to be aged 25–35 years and to have a body mass index of 18.5–24.9 kg/m2.29 At the time of the study, all participants were free of rhinitis, influenza, or any other symptom of upper respiratory infection. Participants were excluded if they had diabetes mellitus or a known allergy to any of the test ingredients, or if they were using any anti-inflammatory, analgesic, antiallergy, antidepressant medication, or multivitamins. Participants received oral and written information about the experimental procedures, and written consent was obtained before administration of any treatment.
Study description
Ten male and ten female study subjects who had fasted overnight for approximately 12–16 hours and met the aforementioned inclusion criteria were treated at 8 am with placebo, which consisted of an empty capsule on day 1; a single dose of 75 g of glucose in water on day 2; and 50 mg of encapsulated Dermaval followed 15 minutes later by 75 g of glucose in water on day 3. Prior to treatment, blood was collected from the subjects at baseline. Treatment was initiated at 8 am in the morning and continued for 2 hours. Blood was collected at 60 and 120 minutes on days 1 and 2, and at 60 and 120 minutes after the glucose load on day 3. In all cases, subjects fasted for 12 hours prior to the first blood collection. Other than the study materials, patients had no intake by mouth during the study period and remained calm and resting. One hundred milliliters of blood were collected by finger puncture and placed in Safe-T-Fill® lithium-heparin capillary blood collection tubes (Ram Scientific Inc., Yonkers, NY, USA) at baseline. Subsequent samples were collected 60 and 120 minutes after treatment. Glucose was measured using an Accu-Chek® Compact Plus glucometer and Accu-Chek test strips (Roche Diagnostics, Indianapolis, IN, USA). After blood was collected for the HNE assays, the fingers were wiped clean and a glucose test was performed on fresh blood. Glucose was read according to the instructions provided by the manufacturer.
Materials
Dermaval is a proprietary formulation of vegetable and fruit components and protected by a provisional patent. The Dermaval tested in this study was provided by FutureCeuticals Inc., (Momence, IL, USA). Punicalagin A and B and chlorogenic acid standards (neochlorogenic acid 3-CQA, chlorogenic acid 5-CQA, cryptochlorogenic acid 4-CQA, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid) were manufactured by PhytoLab (Vestenbergsgreuth, Germany) and purchased from Cerilliant Corporation (Round Rock, TX, USA). Authentic standards of quercetin, kaempferol, isorhamnetin, and rutin (quercetin 3-O-rutinoside) were purchased from Sigma-Aldrich (St Louis, MO, USA), and kaempferol-3-O-rutinoside (nicotiflorin) and isorhamnetin 3-O-rutinoside (narcissin) were purchased from Extrasynthese (Genay, France). The ascorbic acid standard was obtained from United States Pharmacopeia (Rockville, MD, USA). Dulbecco’s phosphate-buffered saline, D-(+)-glucose, and water were purchased from Sigma Chemical Company (St Louis, MO, USA). Protein Low Binding microtubes were obtained from Eppendorf (Hauppauge, NY, USA). An RC DC Protein Assay Kit II was purchased from Bio-Rad Inc., (Palo Alto, CA, USA). Empty gelatin capsules were obtained from Capsuline Inc., (Pompano Beach, FL, USA). An InnoZyme™ human neutrophil elastase (EC 3.4.21.37) immunocapture activity assay kit was sourced from Calbiochem® (EMD Millipore Corporation, Billerica, MA, USA) and from Cayman Chemical Company (Ann Arbor, MI, USA). For total HNE detection, ELA2 (EC 3.4.21.37) enzyme-linked immunosorbent assay kits were obtained from Abnova Corporation (Taipei City, Taiwan). Lancets were purchased from Medlance® (Ozorkow, Poland). The placebo treatments consisted of empty gelatin capsules obtained from Capsuline Inc.
Detection and quantification of HNE
Heparin plasma was isolated from collected blood samples by centrifuging at 1,000 × g for 10 minutes. Total HNE was detected using a colorimetric quantitative sandwich enzyme-linked immunosorbent assay kit following the instructions provided in the kit. Final reactions were measured using a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) and HNE concentrations were determined based on a standard curve as recommended by the manufacturer. Total HNE activity was determined using HNE immunocapture activity assay kits.
Chemical analysis of Dermaval
Punicalagin analysis
The punicalagin content was measured by a modified high-performance liquid chromatography (HPLC) method developed by Seeram et al30 and Lu et al.31 Two milligrams of the punicalagin standard was dissolved in 5 mL of phosphoric acid for a final concentration of 0.5 volume-volume percent (0.5% v/v), sonicated for 8 minutes, cooled to room temperature, and then diluted to 10 mL with phosphoric acid. An aliquot of the prepared standard was transferred to an autosampler vial for HPLC analysis. One gram of the powdered Dermaval sample was dissolved in 25 mL of phosphoric acid (0.5% v/v), sonicated for 8 minutes, cooled to room temperature, and diluted to 50 mL with phosphoric acid (0.5% v/v). The sample was then centrifuged at a relative centrifugal force of 3,900 × g at 4°C for 20 minutes, and the supernatant was analyzed by HPLC on an Agilent 1100 system (Agilent Technologies, Palo Alto, CA, USA), with a photodiode array detector controlled by Chemstation® chromatography software. Samples were eluted through a Zorbax SB-C18 reversed-phase column (5 μm, 4.6 × 150 mm, Agilent Technologies) using mobile phases consisting of 0.5% v/v phosphoric acid (mobile phase A) and methanol (mobile phase B). The gradient system began with 100% of mobile phase A and 0% of mobile phase B (flow rate 1.0 mL per minute), and after injection (10 μL), this was changed to 100% mobile phase B linearly after 20 minutes. After 25 minutes, it was changed again to 100% mobile phase A and 0% mobile phase B over 5 minutes.
Chlorogenic acid analysis
The modified HPLC method for chlorogenic acids developed by Schrader et al was used for this analysis.32 Two milligrams of each chlorogenic acid standard (neochlorogenic acid 3-CQA, chlorogenic acid 5-CQA, cryptochlorogenic acid 4-CQA, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid) were dissolved in 5 mL of methanol (50% v/v with water). The standard preparation was sonicated for 10 minutes, cooled to room temperature, and diluted to 10 mL with methanol. One gram of the powdered Dermaval sample was diluted in 50 mL of methanol (50% v/v with water), sonicated for 10 minutes, cooled to room temperature, and diluted to 100 mL with methanol. The sample was then filtered using a GHP syringe filter (0.45 μm) prior to HPLC analysis, which was performed on an LC-20 HPLC system (Shimadzu, Kyoto, Japan) consisting of a binary pump with a degasser, a refrigerated autosampler, and a photodiode array detector controlled by LabSolutions chromatographic software. The column used was a Luna C18 (2) reversed-phase column (5 μm, 4.6 × 150 mm) from Phenomenex (Torrance, CA, USA). The initial conditions were 95% acetic acid (2% v/v with water) for mobile phase A and 5% acetonitrile for mobile phase B, at a flow rate of 1.0 mL per minute. The chlorogenic acids were detected by monitoring at 325 nm on the photodiode array detector. The mobile phase gradient program changed linearly to 75% mobile phase A over 60 minutes and then changed to the initial condition (95% mobile phase A) over 30 minutes.
Flavonoid analysis
Two milligrams of each flavonoid were diluted in 5 mL of methanol, sonicated for one hour at room temperature, and centrifuged at 5,900 rpm prior to immediate HPLC analysis. One gram of the powdered Dermaval sample was dissolved in 50 mL of methanol, sonicated for 10 minutes, cooled to room temperature, and diluted to 100 mL with water. The sample was then filtered using a GHP syringe filter (0.45 μm) prior to analysis. Kaempferol, quercetin, and rutin analyses were performed by the HPLC method33 on a Waters 2690 separation module (Waters Corporation, Milford, MA, USA) and a Waters 996 photodiode array detector controlled by Waters Empower 2 chromatographic software (Build Number 2154, Database version 6.10.01). The column used was a Luna C18 (2) reversed-phase column (5 μm, 4.6 × 150 mm) from Phenomenex. The initial conditions were 95% acetic acid (2% v/v with water) and 5% acetonitrile, using a flow rate of 1.0 mL per minute (detection at 325 nm). The mobile phase gradient program was changed linearly to 75% mobile phase A over 60 minutes and then changed to the initial condition (95% mobile phase A) over 30 minutes. Isorhamnetin, nicotiflorin, and narcissin were analyzed on an 1100 HPLC system (Agilent Technologies) with 5 μm Phenyl-Hexyl column (250 × 4.6 mm) from Phenomenex. The gradient profile for the separation of flavonoids was formed using solvent A (89% water, 9% acetonitrile, and 2% acetic acid) and solvent B (20% water and 80% acetonitrile). The gradient method started at 1 mL per minute with 100% solvent A for 25 minutes and was then linearly changed to 100% solvent B over 15 minutes.34
Ascorbic acid analysis
Two milligrams of ascorbic acid were dissolved in 5 mL of potassium phosphate buffer (0.2 M KH2PO4 pH 2.4) and diluted to 10 mL with this buffer. The powdered sample was weighed and added directly into a 50 mL volumetric flask (about 4 mg/mL). Next, 25 mL of potassium phosphate buffer (0.2 M KH2PO4, pH 2.4) was added and mixed. The sample was sonicated for 10 minutes, cooled to room temperature, diluted to volume with potassium phosphate buffer, and mixed. The sample was then filtered using a GHP syringe filter (0.45 μm) and transferred to an autosampler vial for analysis. HPLC analysis of ascorbic acid was performed on an Agilent 1100 HPLC system with a refrigerated autosampler and a photodiode array detector controlled by Chemstation® (Agilent Technologies, Santa Clara, CA, USA) chromatography software. The samples were eluted through an Allure organic acids column (5 μm, 4.6 × 150 mm, from Restek Corporation, Bellefonte, PA, USA) under the initial conditions of 100% potassium phosphate buffer (0.2 M KH2PO4, pH 2.4) at a flow rate of 1.0 mL per minute with a column temperature of 30°C (detection at 214 nm).
Statistical analysis
HNE levels were normalized using time zero as the baseline. Statistical analysis was performed using the commercially available GraphPad® statistical software (Graphpad Software Inc., La Jolla, CA, USA). Descriptive statistics are presented by the mean ± standard error. Plasma HNE levels at 60 and 120 minutes after treatment were compared within the experimental groups with baseline and between experimental groups using a one-way analysis of variance with Tukey’s post hoc analysis when a significant F-ratio was observed. Statistical significance was set at P<0.05.
Results
We identified and quantified the major phytochemicals present in 1 g of Dermaval (Table 1). Punicalagins were the most abundant phytochemical present in the sample, at 202.26 mg/g. The next most abundant phytochemicals, chlorogenic acids, quercetin, and ascorbic acid, were an order of magnitude less abundant at 15.51, 13.98, and 8.71 mg/g, respectively. The other components each represented less than 1 mg/g of Dermaval.
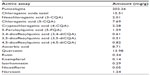  | Table 1 Major phytochemicals identified in Dermaval™ |
The specific activity of HNE is shown in Figure 1. There was no difference in HNE activity among the untreated individuals over the 2 hours of the study. Treatment with glucose significantly increased HNE activity over the initial baseline value at both time points (171%±25% at 60 minutes after glucose ingestion versus baseline, P<0.028; 186%±51% at 120 minutes after glucose ingestion versus baseline, P<0.045). Participants who consumed Dermaval prior to glucose did not show an increase in HNE activity. Mean HNE activity was 100%±8% and 88%±10% of baseline at 60 and 120 minutes after glucose ingestion, respectively. Treatment with Dermaval prior to glucose resulted in HNE activity similar to that of fasting alone and significantly lower than glucose treatment alone (P<0.005 60 minutes after glucose ingestion and P<0.039 120 minutes after glucose ingestion versus glucose only).
Blood glucose levels were measured at all time points in all three experimental groups to ensure that none of the study subjects experienced hypoglycemia after overnight fasting and to verify whether ingestion of Dermaval affected blood glucose levels. As seen in Figure 2, there was no difference in blood glucose levels between the group treated with glucose and the group treated with Dermaval and glucose.
Discussion
We demonstrated that a modest, single dose of glucose can increase HNE activity in humans during the first 60 minutes after ingestion. We then exploited this effect to test putative elastase inhibitors. We report here for the first time that a single 50 mg dose of Dermaval can prevent a glucose-induced increase in HNE activity. Dermaval did not directly affect blood glucose levels.
The inhibitory effect of Dermaval on HNE activity did not correspond to a simultaneous reduction in blood glucose levels under these experimental conditions. It is unclear as to how Dermaval affects HNE activity. Our hypothesis is that this phytochemical preparation indirectly inhibits elastase activity. We are currently testing the hypothesis that Dermaval may affect blood levels of metalloproteinases, tissue inhibitors of metalloproteinases, proinflammatory cytokines, and chemokines under these experimental conditions. If confirmed, Dermaval may contribute to curtailing the proinflammatory state caused by high sugar consumption. Excessive HNE activity is thought to play a role in various pathologic processes.
Increased levels of HNE activity have been measured in pneumonia,35 cystic fibrosis,36 and complications associated with balloon angioplasty.37 A study by Talukdar et al38 showed that neutrophil elastase secreted by neutrophils contributes to insulin resistance induced by a high-fat diet. HNE inhibition is recognized as an important molecular target for preventing HNE-mediated pathologies and health problems.39 Several natural nondrug substances have been shown to inhibit HNE activity.11,12
We report that Dermaval possesses acute anti-HNE activity during the first hour after treatment, presumably due to one or more of its constituent phytochemicals. Quercetin, for example, can suppress HNE transcription and expression;14 however, its role as a direct inhibitor is less clearly understood. A 50 mg dose of Dermaval contains rather low levels of these phytochemicals, yet the current clinical data would suggest that it potently modulates HNE activity. It is conceivable that more than one constituent possesses anti-HNE activity or that the constituents work together synergistically.
In summary, a single 50 mg dose of Dermaval was shown to inhibit glucose-induced HNE activity in human healthy subjects. Further investigations are needed to verify whether Dermaval inhibits HNE activity directly or indirectly. In addition, the results justify testing of Dermaval for skin health following treatment over a longer period of time.
Acknowledgments
We express our gratitude to John Hunter and Brad Evers (FutureCeuticals Inc.) for their comments and suggestions in the preparation of this paper. We would like to thank Michael Sapko for his help in editing the manuscript.
Disclosure
This study was funded by FutureCeuticals Inc. The authors report no other conflicts of interest in this work.
References
McDonald A. ExplorEnz. The Enzyme Database. 2012. Available from: http://www.enzyme-database.org/newenz.php?rb=on&fd=2012-10-01&ld=2012-12-31. Accessed November 1, 2013. | |
Takahashi H, Nukiwa T, Yoshimura K, et al. Structure of the human neutrophil elastase gene. J Biol Chem. 1988;263:14739–14747. | |
Bode W, Wei AZ, Huber R, Meyer E, Travis J, Neumann S. X-ray crystal structure of the complex of human leukocyte elastase (PMN elastase) and the third domain of the turkey ovomucoid inhibitor. EMBO J. 1986;5:2453–2458. | |
Thusberg J, Vihinen M. Bioinformatic analysis of protein structure-function relationships: case study of leukocyte elastase (ELA2) missense mutations. Hum Mutat. 2006;27:1230–1243. | |
Schmelzer CE, Jung MC, Wohlrab J, Neubert RH, Heinz A. Does human leukocyte elastase degrade intact skin elastin? FEBS J. 2012;279:4191–4200. | |
Zhu YK, Liu XD, Skold CM, et al. Synergistic neutrophil elastase-cytokine interaction degrades collagen in three-dimensional culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L868–L878. | |
Takeuchi H, Gomi T, Shishido M, Watanabe H, Suenobu N. Neutrophil elastase contributes to extracellular matrix damage induced by chronic low-dose UV irradiation in a hairless mouse photoaging model. J Dermatol Sci. 2010;60:151–158. | |
Imokawa G. Mechanism of UVB-induced wrinkling of the skin: paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J Investig Dermatol Symp Proc. 2009;14:36–43. | |
Tsukahara K, Takema Y, Moriwaki S, et al. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J Invest Dermatol. 2001;117:671–677. | |
Siedle B, Hrenn A, Merfort I. Natural compounds as inhibitors of human neutrophil elastase. Planta Med. 2007;73:401–420. | |
Ryoo I-J. Hydoroxyhibiscone A, a novel human neutrophil elastase inhibitor from Hibiscus syriacus. J Microbiol Biotechnol. 2010;20:1189–1191. | |
Sivamani P, Singaravelu G, Thiagarajan V, Jayalakshmi T, Ramesh Kumar G. Comparative molecular docking analysis of essential oil constituents as elastase inhibitors. Bioinformation. 2012;8:457–460. | |
Jimenez F, Mitts TF, Liu K, Wang Y, Hinek A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J Invest Dermatol. 2006;126:1272–1280. | |
Li N, Li Q, Zhou XD, Kolosov VP, Perelman JM. The effect of quercetin on human neutrophil elastase-induced mucin5 AC expression in human airway epithelial cells. Int Immunopharmacol. 2012;14:195–201. | |
Kwak HM, Jeon SY, Sohng BH, et al. beta-Secretase (BACE1) inhibitors from pomegranate (Punica granatum) husk. Arch Pharm Res. 2005;28:1328–1332. | |
Lee WL, Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. | |
Doring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150(6 Pt 2):S114–S117. | |
Camussi G, Tetta C, Bussolino F, Baglioni C. Synthesis and release of platelet-activating factor is inhibited by plasma alpha 1-proteinase inhibitor or alpha 1-antichymotrypsin and is stimulated by proteinases. J Exp Med. 1988;168:1293–1306. | |
Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986;83:6692–6696. | |
Dijkman JH. Antileucoprotease in the airways and emphysema. Monaldi Arch Chest Dis. 1995;50:383–387. | |
Remold-O’Donnell E, Chin J, Alberts M. Sequence and molecular characterization of human monocyte/neutrophil elastase inhibitor. Proc Natl Acad Sci U S A. 1992;89:5635–5639. | |
Cooley J, Takayama TK, Shapiro SD, Schechter NM, Remold-O’Donnell E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry. 2001;40:15762–15770. | |
Schalkwijk J, Wiedow O, Hirose S. The trappin gene family: proteins defined by an N-terminal transglutaminase substrate domain and a C-terminal four-disulphide core. Biochem J. 1999; 340(Pt 3):569–577. | |
Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. | |
Lin B, Ginsberg MD, Busto R, Li L. Hyperglycemia triggers massive neutrophil deposition in brain following transient ischemia in rats. Neurosci Lett. 2000;278:1–4. | |
de Souza Ferreira C, Araujo TH, Angelo ML, et al. Neutrophil dysfunction induced by hyperglycemia: modulation of myeloperoxidase activity. Cell Biochem Funct. 2012;30(7):604–610. | |
Vantyghem MC, Haye S, Balduyck M, Hober C, Degand PM, Lefebvre J. Changes in serum amylase, lipase and leukocyte elastase during diabetic ketoacidosis and poorly controlled diabetes. Acta Diabetol. 1999;36(1–2):39–44. | |
Choi HJ, Yun HS, Kang HJ, et al. Human transcriptome analysis of acute responses to glucose ingestion reveals the role of leukocytes in hyperglycemia-induced inflammation. Physiol Genomics. 2012;44:1179–1187. | |
Borecki IB, Higgins M, Schreiner PJ, et al. Evidence for multiple determinants of the body mass index: the National Heart, Lung, and Blood Institute Family Heart Study. Obes Res. 1998;6:107–114. | |
Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separation and Purification Technology. 2005;41:49–55. | |
Lu J, Ding K, Yuan Q. Determination of punicalagin isomers in pomegranate husk. Chromatographia. 2008;68:303–306. | |
Schrader K, Kiehne A, Engelhardt UH, Maier HG. Determination of chlorogenic acids with lactones in roasted coffee. J Sci Food Agric. 1996;71:392–398. | |
Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z. Phenols, proanthocyanidins, flavones, and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. | |
Fuentes-Alventosa JM, Jaramillo S, Rodríguez-Gutíerrez G, et al. Flavonoid profile of green asparagus genotypes. J Agric Food Chem. 2008;56:6977–6984. | |
Tagami T, Kushimoto S, Yamamoto Y, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care. 2010;14:R162. | |
Jones MM, Seilheimer DK, Pier GB, Rossen RD. Increased elastase secretion by peripheral blood monocytes in cystic fibrosis patients. Clin Exp Immunol. 1990;80:344–349. | |
Barolet AW, Nili N, Cheema A, et al. Arterial elastase activity after balloon angioplasty and effects of elafin, an elastase inhibitor. Arterioscler Thromb Vasc Biol. 2001;21:1269–1274. | |
Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. | |
Tremblay GM, Janelle MF, Bourbonnais Y. Anti-inflammatory activity of neutrophil elastase inhibitors. Curr Opin Investig Drugs. 2003;4:556–565. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


