Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Depressive disorders co-existing with Addison–Biermer anemia – case report
Authors Just M, Kozakiewicz M
Received 19 February 2015
Accepted for publication 19 March 2015
Published 30 April 2015 Volume 2015:11 Pages 1145—1148
DOI https://doi.org/10.2147/NDT.S83137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Mark Jean Just,1 Mariusz Kozakiewicz2
1Department of General and Endocronological Surgery, Piekary Medical Centre, Piekary Slaskie, 2Department of Food Chemistry Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Torun, Poland
Background: Anemia is a disease that can co-exist with depression, other mental disorders, or somatic diseases. Anemia can imitate symptoms of depression, while depression symptoms can mask concurring symptoms of anemia.
Case presentation: I am presenting a case of a 48-year-old woman with Addison–Biermer anemia, with co-existing mood disorders. The clinical analysis of the presented patient’s history indicates diagnostic problems and a need for a detailed analysis of drug-related complications that occurred during previous treatment, eg, in the form of neuroleptic malignant syndrome.
Conclusion: The presented case report contains valuable guidelines that can be of assistance in diagnostics and treatment of patients treated for mental disorders, who are also diagnosed with somatic diseases.
Keywords: anemia, autoimmune diseases, depression, neuroleptic malignant syndrome
Introduction
Pernicious anemia, also called Addison–Biermer anemia, is an autoimmune disease most often occurring in people with A blood type, and caused by vitamin B12 absorption disturbances (Figure 1). In the initial clinical presentation, symptoms of this anemia, including apathy, psychomotor retardation, lack of energy, fatigability, or excessive sleepiness, can indicate a possibility of concurring depression.1,2 Furthermore, symptoms of depression can overlap with somatic symptoms of anemia, further complicating the clinical presentation, and therefore, the treatment of underlying disease, or even persisting when anemia symptoms have resolved. Anemia was identified in 10% of patients with depression. Patients particularly susceptible to stress and suffering from anxious personality disorders concomitant with somatic diseases belong to a risk group prone to depressive disorders and anemia. Depression can develop against a background of a chronic stress reaction related to the somatic disease.3 Chronic stress can also lead to development of conversion disorders, with partial or complete loss of correct integration of past memories with a sense of own identity.4,5 Increased conversion or depression symptoms in patients with Addison–Biermer anemia can represent a difficult diagnostic and treatment challenge to hematology specialists, general practitioners, and psychiatrists. Treatment of depression and conversion symptoms should combine pharmaco- and psychotherapy.6 For severe depression, treatment with anti-depressive drugs alone may be insufficient, requiring additional inclusion of atypical neuroleptics.7 It is worth noting that in people suffering from depression and with a positive history of somatic diseases, such as Addison–Biermer anemia or endocrine diseases, the neuroleptic malignant syndrome (NMS) is more likely to occur following neuroleptic treatment, than in people without somatic diseases.
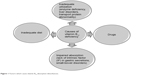  | Figure 1 Factors which cause vitamin B12 absorption disturbances. |
Case presentation
I am presenting a clinical case of a 48-year-old patient, with A blood type, admitted to the surgical ward due to anemia. It is known from the interview that the patient was previously pharmacologically treated for hypothyroidism, but has not taken medications for several years. The patient felt weak, had concentration problems, and reduced tolerance of physical exercise for approximately 1 month. In physical examination, pale skin and mucous membranes were found.
The patient underwent the following examinations: ultrasound scan of the abdominal cavity, gastroscopy, colonoscopy, computed tomography of the abdominal cavity, and ultrasound scan of the thyroid. The results showed no changes. Laboratory tests conducted on admission day revealed: white blood corpuscules 4.56 K/μL; lymphocytes 2.41; red blood corpuscules 3.00 M/μL; hemoglobin 8.74 g/dL; mean corpuscular volume 81.8 fl; mean corpuscular hemoglobin 28.7 pg; mean corpuscular hemoglobin concentration 35.7 g/dL; platelets 158 K/μL; vitamin B12 level 120 pg/mL (reference value 180–900 pg/mL); iron level 160 μg/dL (reference value 60–160 μg/dL). Thyroid hormone levels for free thyroid hormone – triiodothyronine, free thyroid hormone – thyroxine, and thyroid-stimulating hormone levels were within the reference values.
Only in the tenth day of her hospitalization, the patient disclosed that in the past she had underwent psychiatric treatment for depression and conversion disorders. She was afraid of mental disease-related stigma. Five years before current hospitalization, the patient had had NMS due to treatment with olanzapine for psychotic depression. During psychiatric consultation, the patient admitted she had not taken neuroleptics for about a year. She decided to discontinue the therapy. For the last 3 months, the patient has complained about deteriorating psychical condition revealing symptoms such as: low mood, apathy, anhedonia, anxiety, memory and concentration problems, sleep disturbances, and suicidal thoughts. The patient was diagnosed with severe depression without psychotic symptoms and with Addison–Biermer anemia. Depression diagnosis was based on clinical assessment of the patient and followed by performing Beck Depression Inventory in which the patient received 26 scores.8 Pharmacological treatment was commenced, with venlafaxine, 225 mg/day, and valproic acid, 1,000 mg/day. After 4 weeks of treatment, mirtazapine was added at a dose of 30 mg/day. Mood and psychomotor drive were partially improved after 10 weeks of treatment. Following 4 weeks of treatment, Beck Depression Inventory was repeated. This time the patient received 19 scores, which indicate improvement of the patient’s mental status.
Simultaneously, vitamin B12 at a dose of 1,000 μg/d was commenced. The vitamin was administered intramuscularly daily for 10 days, then three times a week for 3 weeks, and then twice a week for the next 3 weeks. At the next stage of the treatment folic acid was added at a dose of 15 μg/d and after 10 weeks of therapy, anemia symptoms disappeared.
Discussion
When suffering from somatic symptoms of anemia or symptoms resulting from a thyroid function disorder, patients usually visit their general practitioner, hematologist, endocrinologist, or surgeon. The most important task of the doctor is to establish whether some or all of presented symptoms are of an organic origin, or not. The organic cause requires a clinical intervention, eg, in the form of pharmacological or surgical treatment.9 However, it should be remembered that the initial organic disease may be accompanied by concurrent secondary mental disorders.10,11 It is recommended to perform screening laboratory tests in patients with depression, excluding anemia and hypothyroidism, such as: complete blood count, serum iron level, total iron binding capacity, unsaturated iron binding capacity, serum vitamin B12 level, and thyroid-stimulating hormone. In patients belonging to a risk group susceptible to depression, it is recommended to determine oxidative stress markers, eg, chemokines, cytokines. These markers may constitute an early predictor of affective disorders.12 When the organic cause is excluded, or when concurrent symptoms of mental disorders are observed, then the basic medical procedure involves psychiatric consultation for correct diagnosis and treatment initiation.
Addison–Biermer anemia can co-exist with other somatic diseases or accompany mental disorders (depression, anxiety disorders, conversion disorders).13
It is a strong predictor of adverse clinical incidents related to cardiac ischemia. Anemia is associated with higher short- and long-term risk of death in coronary disease patients with a stable form of that disease, heart infarction with ST segment elevation, and with acute coronary syndromes without ST segment elevations, as well as in patients after coronary vessel interventions. Anemia is an important mortality and morbidity risk factor in chronic kidney failure. A decrease in hemoglobin (Hb) by 1 g/dL is associated with 14%–18% increase in mortality in a dialysis patient population, while Hb level decrease below 8 g/dL doubles the risk of death versus patients with Hb of 10–11 g/dL.
In depression, concurrence of somatic diseases, such as Addison–Biermer anemia or thyroid hormone disturbances can delay therapy, for example, using electroconvulsive therapy in treatment of severe depression.14
In treatment of depression, delayed use of electroshocks can result in exacerbation of depression and contribute to the increased risk of suicide.15 Moreover, in patients treated with atypical neuroleptics there is a risk of NMS. According to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), it is possible to diagnose NMS when muscle rigidity and fever are found with at least two of the following symptoms concurring: diaphoresis, dysphagia, incontinence, changes in level of consciousness, mutism, tachycardia, increased or labile blood pressure, leukocytosis, and increased CK levels, indicating muscle injury. Other criteria proposed by Levenson include “major symptoms” (fever, muscle rigidity, and increased CK levels) and “minor symptoms” (tachycardia, variable blood pressure, tachypnea, changes in level of consciousness, diaphoresis, and leukocytosis). The NMS diagnosis is justified in cases with all “major symptoms” present, or when two of them are accompanied by at least two “minor symptoms”.16 NMS can also develop in patients with positive history of somatic diseases, such as thyroid disorders, anemia, and neurological diseases, or in patients without chronic diseases.17
Furthermore, patients who suffered from NMS caused by olanzapine are predisposed to NMS in the future also after administration of other drugs affecting the dopaminergic system.18,19 Metabolic changes secondary to hypothyroidism or Addison–Biermer disease, particularly in the dopaminergic pathways within the central nervous system, can also predispose to the development of NMS.20
Conclusion
In treatment of depressive disorders, a possibility of concurrence of somatic disorders in the same patient should also be considered. Taking into consideration the fact that mental disorders often represent prodromal signs of various somatic diseases, it is necessary to remember that additional tests and evaluation of the somatic status are of particular importance in psychiatric patients. It is worth noting that these patients focus on their emotional experience and ignore their somatic problems.
Acknowledgments
The work was carried out at Department of General Surgery, Municipal Hospital in Piekary Slaskie, Poland. Professional language editing has been performed by native speaker.
Author contributions
Both authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Andrès E. Oral cobalamin (vitamin B12) therapy in pernicious anemia. Autoimmun Rev. 2014;13(7):778. | ||
Lacka K, Maciejewski A, Florczak-Wyspiańska J. Coexistence of Addison-Biermer’s disease with autoimmune thyroiditis – case report. Pol Merkur Lekarski. 2013;34(199):40–44. | ||
Lever-van Milligen BA, Vogelzangs N, Smit JH, Penninx BW. Hemoglobin levels in persons with depressive and/or anxiety disorders. J Psychosom Res. 2014;76(4):317–321. | ||
Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM. Sex differences in the chronic mild stress model of depression. Behav Pharmacol. 2014;25(5–6):372–383. | ||
Szota A, Oglodek E, Araszkiewicz A. A female patient with depression and conversion disorder following brain tumor surgery. Aust N Z J Psychiatry. 2013;47(12):1213–1214. | ||
Cuijpers P. Combined pharmacotherapy and psychotherapy in the treatment of mild to moderate major depression? JAMA Psychiatry. 2014;71(7):747–748. | ||
Pitchot W, Scantamburlo G, Souery D. Des antipsychotiques dans la dépression, [Antipsychotics in depression]. Rev Med Liege. 2013;68(10): 521–526. French. | ||
Mandelli L, Nearchou FA, Vaiopoulos C, et al. Neuroticism, social network, stressful life events: Association with mood disorders, depressive symptoms and suicidal ideation in a community sample of women. Psychiatry Res. 2015;226(1):38–44. | ||
Lorentzen K, Kjær B, Olsen KS. Behandling af svær anæmi hos patienter, der afviser blodtransfusion, [Treatment of severe anaemia in patients who refuse blood transfusion]. Ugeskr Laeger. 2014;176(7). Danish. | ||
Pol RR, Howard MR. A man with anemia and a change in personality. Blood. 2014;123(12):1784. | ||
Green MW, Elliman NA. Are dieting-related cognitive impairments a function of iron status? Br J Nutr. 2013;109(1):184–192. | ||
Rybka J, Kędziora-Kornatowska K, Banaś-Leżańska P, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–194. | ||
Duda-Król WB, Kusz-Rynkun A, Pietrzak J, Soszka A. Częstość występowania rodzinnej niedokrwistości Addisona-Biermera, [Family incidence of Addison-Biermer anemia]. Wiad Lek. 2005;58(11–12): 685–687. Polish. | ||
Szupień E, Ositek B, Pniewski J. Trudności diagnostyczne w przypadku podostrej paraplegii u kobiety z choroba Addisona-Biermera, [Difficulties in the diagnosis in the case of subacute paraplegia in a woman with Addison-Biermer disease]. Neurol Neurochir Pol. 2004;38(5):431–436. Polish. | ||
Oglodek E, Szota A, Araszkiewicz A. Electroconvulsive therapy in a patient with drug-resistant depression and thyroid hormone imbalance. Aust N Z J Psychiatry. 2014;48(1):96–97. | ||
Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry. 1985; 142(10):1137–1145. | ||
Szota A, Ogłodek E, Araszkiewicz A. Fever development in neuroleptic malignant syndrome during treatment with olanzapine and clozapine. Pharmacol Rep. 2013;65(2):279–287. | ||
Oglodek E, Szota A, Araszkiewicz A. Olanzapine-induced neuroleptic malignant syndrome after 10 years of treatment. Aust N Z J Psychiatry. 2013;47(10):972. | ||
Szota A, Oglodek E, Araszkiewicz A. Bipolar disorder: Mixed episodes concomitant with gambling addiction. Aust N Z J Psychiatry. 2013;48(6):586–587. | ||
Kosehasanogullari SG, Akdede B, Akvardar Y, Akan M, Tunca Z. Neuroleptic malignant syndrome caused by combination of risperidone and lithium in a patient with multiple medical comorbidities. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1147–1148. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
