Back to Journals » Drug Design, Development and Therapy » Volume 9
Danusertib, a potent pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle arrest and programmed cell death and inhibits epithelial to mesenchymal transition involving the PI3K/Akt/mTOR-mediated signaling pathway in human gastric cancer AGS and NCI-N78 cells
Authors Yuan C, Zhou Z, Yang Y, He Z, Zhang X, Wang D, Yang T, Pan S, Chen X, Zhou S
Received 26 September 2014
Accepted for publication 22 October 2014
Published 2 March 2015 Volume 2015:9 Pages 1293—1318
DOI https://doi.org/10.2147/DDDT.S74964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Chun-Xiu Yuan,1,2 Zhi-Wei Zhou,2,3 Yin-Xue Yang,4 Zhi-Xu He,3 Xueji Zhang,5 Dong Wang,6 Tianxing Yang,7 Si-Yuan Pan,8 Xiao-Wu Chen,9 Shu-Feng Zhou2
1Department of Oncology, General Hospital, Ningxia Medical University, Yinchuan, People’s Republic of China; 2Department of Pharmaceutical Science, College of Pharmacy, University of South Florida, Tampa, FL, USA; 3Guizhou Provincial Key Laboratory for Regenerative Medicine, Stem Cell and Tissue Engineering Research Center and Sino-US Joint Laboratory for Medical Sciences, Guiyang Medical University, Guiyang, 4Department of Colorectal Surgery, General Hospital, Ningxia Medical University, Yinchuan, 5Research Center for Bioengineering and Sensing Technology, University of Science and Technology Beijing, 6Cancer Center, Daping Hospital and Research Institute of Surgery, Third Military Medical University, Chongqing, People’s Republic of China; 7Department of Internal Medicine, University of Utah and Salt Lake Veterans Affairs Medical Center, Salt Lake City, UT, USA; 8Department of Pharmacology, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 9Department of General Surgery, The First People’s Hospital of Shunde, Southern Medical University, Shunde, People’s Republic of China
Abstract: Gastric cancer is the second leading cause of cancer-related death worldwide, with a poor response to current chemotherapy. Danusertib is a pan-inhibitor of the Aurora kinases and a third-generation Bcr-Abl tyrosine kinase inhibitor with potent anticancer effects, but its antitumor effect and underlying mechanisms in the treatment of human gastric cancer are unknown. This study aimed to investigate the effects of danusertib on cell growth, apoptosis, autophagy, and epithelial to mesenchymal transition and the molecular mechanisms involved in human gastric cancer AGS and NCI-N78 cells. The results showed that danusertib had potent growth-inhibitory, apoptosis-inducing, and autophagy-inducing effects on AGS and NCI-N78 cells. Danusertib arrested AGS and NCI-N78 cells in G2/M phase, with downregulation of expression of cyclin B1 and cyclin-dependent kinase 1 and upregulation of expression of p21 Waf1/Cip1, p27 Kip1, and p53. Danusertib induced mitochondria-mediated apoptosis, with an increase in expression of proapoptotic protein and a decrease in antiapoptotic proteins in both cell lines. Danusertib induced release of cytochrome c from the mitochondria to the cytosol and triggered activation of caspase 9 and caspase 3 in AGS and NCI-N78 cells. Further, danusertib induced autophagy, with an increase in expression of beclin 1 and conversion of microtubule-associated protein 1A/1B-light chain 3 (LC3-I) to LC3-II in both cell lines. Inhibition of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and p38 mitogen-activated protein kinase pathways as well as activation of 5' AMP-activated protein kinase contributed to the proautophagic effect of danusertib in AGS and NCI-N78 cells. SB202191 and wortmannin enhanced the autophagy-inducing effect of danusertib in AGS and NCI-N78 cells. In addition, danusertib inhibited epithelial to mesenchymal transition with an increase in expression of E-cadherin and a decrease in expression of N-cadherin in both cell lines. Taken together, danusertib has potent inducing effects on cell cycle arrest, apoptosis, and autophagy, but has an inhibitory effect on epithelial to mesenchymal transition, with involvement of signaling pathways mediated by PI3K/Akt/mTOR, p38 mitogen-activated protein kinase, and 5' AMP-activated protein kinase in AGS and NCI-N78 cells.
Keywords: danusertib, gastric cancer, Aurora kinase, apoptosis, autophagy, epithelial to mesenchymal transition
Introduction
Gastric cancer is one of the most common cancers worldwide, ranking fifth as the most common malignancy after lung, breast, colorectum, and prostate cancer.1,2 Gastric cancer is the second leading cause of cancer-related death.2,3 There were 952,000 new cases of gastric cancer worldwide in 2012.4 In the USA, there were about 74,035 people living with gastric cancer in 2011.5,6 In 2014, there have been about 22,220 new cases of gastric cancer, accounting for 1.3% of all new cancer cases. There are about 10,990 gastric cancer-related deaths, accounting for 1.9% of all cancer-related deaths.2,5 In the People’s Republic of China, gastric cancer is the third leading cause of cancer-related death after lung and liver cancer, with 24.71 cases per 100,000 in 2004–2005.2,3 It accounts for 8.8% of cancer-related deaths.2,3 Current treatment for gastric cancer includes surgery, chemotherapy, radiation therapy, chemoradiation, and targeted therapy. However, the prognosis is still quite unsatisfactory, with a 5-year overall survival rate of 28.3%.2 Moreover, chemotherapeutic drugs, including cisplatin, 5-fluorouracil, and trastuzumab, often cause severe side effects and drug resistance, resulting in therapeutic failure in the treatment of gastric cancer. Therefore, there is an urgent need to develop new agents with improved efficacy and reduced side effects to treat gastric cancer.
The Aurora serine-threonine kinases play a critical role in the regulation of mitosis.7 The Aurora kinase family was discovered in 1995, and there are three mammalian Aurora kinases, including Aurora kinase A/B/C (AURKA/B/C).8 They differently localize in cells with differential kinetics of activation and function.7 AURKA and AURKB act as essential regulators of mitosis and the centrosome by polymerizing microfilaments and controlling chromatid segregation, but the function of AURKC is less clear.7 Aurora kinases are key regulators of cell division,9 controlling entry into mitosis, centrosome function, chromosome assembly, and segregation.10 Deregulation of the activity and expression of Aurora kinases can result in aneuploidy and carcinogenesis.11 Recently, increasing evidence implicates the Aurora kinases in the pathogenesis of various types of cancer, drug resistance, and tumor recurrence.7,11 It has been reported that overexpression or activation of AURKA and AURKB promotes development of gastric cancer, with increased gastric cancer cell viability, proliferation, migration, and invasion.12–16 Therefore, targeting Aurora kinase has been considered to be a promising strategy in the treatment of gastric cancer.
A number of Aurora kinase inhibitors have been developed, and have shown variable efficacy at different stages of preclinical and clinical trials (Ding et al, unpublished data, 2014; Yuan et al, unpublished data, 2014).17 Danusertib (Figure 1A), formerly known as PHA-739358, is a potent pan-Aurora kinase inhibitor with activity against all Aurora kinase family members.18 Danusertib has been studied in Phase I and II trials, and has shown considerable therapeutic potential in a wide range of cancers, including advanced solid tumors and leukemias.19,20 However, there is no report on use of danusertib for the treatment of gastric cancer, and the effect of danusertib in gastric cancer is unknown. In the present study, we aimed to explore the anticancer effect and possible mechanisms of danusertib in human gastric cancer AGS and NCI-N78 cells, with a focus on cell cycle distribution, apoptosis, autophagy, and epithelial to mesenchymal transition (EMT).
Materials and methods
Chemicals and reagents
4,6-Diamidino-2-phenylindole was obtained from Invitrogen (Carlsbad, CA, USA). Dulbecco’s Modified Eagle’s Medium and Roswell Park Memorial Institute-1640 medium were sourced from Corning Cellgro Inc (Herndon, VA, USA). Dulbecco’s phosphate-buffered saline, fetal bovine serum, phosphatase inhibitor cocktail, protease inhibitor cocktail, propidium iodide, 50 mmol 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), ethylenediaminetetraacetic acid, RNase A, and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Inc (St Louis, MO, USA). SB202190, 4-(4-fluorophenyl)-2-(4-hydroxyphe nyl)-5-(4-pyridyl)1H-imidazole, a selective inhibitor of p38 mitogen-activated protein kinase (MAPK), used as an autophagy inducer, and wortmannin (WM, a potent, irreversible, and selective phosphatidylinositol 3-kinase [phosphatidylinositol-4 5-bisphosphate 3-kinase] inhibitor and a blocker of autophagosome formation) were obtained from InvivoGen Inc (San Diego, CA, USA). An Annexin V:phycoerythrin (PE) apoptosis detection kit was purchased from BD Biosciences Inc (San Jose, CA, USA). A Cyto-ID® autophagy detection kit was obtained from Enzo Life Sciences Inc (Farmingdale, NY, USA). A Pierce bicinchoninic acid protein assay kit, skim milk, and Western blotting substrate were purchased from Thermo Scientific (Hudson, NH, USA). A polyvinylidene difluoride membrane was obtained from EMD Millipore (Bedford, MA, USA). Primary antibodies against human cyclin B1, cyclin-dependent kinase 1 (CDK1/CDC2/CDKN1), p21 Waf1/Cip1, p27 Kip1, p53, cytochrome c, Bcl-2-like protein 4/Bcl-2-associated X protein (Bax), B-cell lymphoma-extra large (Bcl-xl), B-cell lymphoma 2 (Bcl-2), cleaved (c-)caspase 9, c-caspase 3, p53 upregulated modulator of apoptosis (PUMA), phosphatidylinositol 3-kinase (PI3K), phosphorylated (p-)PI3K/p85 at Tyr458, 5′ AMP-activated protein (AMPK), p-AMPK at Thr172, p38 MAPK, p-p38 MAPK at Thr180/Tyr182, protein kinase B (Akt), p-Akt at Ser473, mammalian target of rapamycin (mTOR), p-mTOR at Ser2448, phosphatase and tensin homolog (PTEN), beclin 1, microtubule-associated protein 1A/1B-light chain 3 (LC3-I), LC3-II, E-cadherin, N-cadherin, vimentin, slug, snail, and β-catenin were all purchased from Cell Signaling Technology Inc (Beverly, MA, USA). The antibody against human β-actin was sourced from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA).
Cell lines and cell culture
Human gastric cancer AGS and NCI-N78 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (AGS cells) and Roswell Park Memorial Institute-1640 medium (NCI-N78 cells) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin in a 5% CO2/95% air humidified incubator at 37°C. AGS and NCI-N78 are the cell lines most commonly used in the investigation of the cancer cell-killing effect and underlying mechanisms of new compounds in human gastric adenocarcinoma. They exhibit remarkable differences in origin and cytogenetics.Danusertib was dissolved in dimethyl sulfoxide at a stock concentration of 50 mM and stored at −20°C. It was freshly diluted to the predetermined concentration with culture medium. The final concentration of dimethyl sulfoxide was 0.05% (v/v). The control cells received vehicle only.
Cell viability assay
The effect of danusertib on the viability of AGS and NCI-N78 cells was examined using the MTT assay as previous described.21 Briefly, AGS and NCI-N78 cells were seeded into a 96-well plate at a density of 8,000 cells/well. After 24 hours of incubation, the cells were treated with danusertib at concentrations ranging from 0.01 to 10 μM for 24 hours with a volume of 100 μL medium. Following treatment with danusertib, 10 μL of MTT stock solution (5 mg/mL) was added to each well and incubated for 4 hours. The medium was then carefully aspirated and 100 μL of dimethyl sulfoxide was added to each well. The plate was placed on a rocker to mix the solution thoroughly for one minute and then incubated at 37°C for 10 minutes. Absorbance was measured using a Synergy H4 Hybrid microplate reader (BioTek Inc, Winooski, VT, USA) at a wavelength of 450 nm. The concentration required for 50% inhibition of growth (IC50) was determined from concentration-response curves obtained in multireplicated experiments by nonlinear regression analysis using GraphPad Prism 6 software (San Diego, CA, USA). The experiment was performed at least three times.
Analysis of cell cycle distribution
The effect of danusertib on the cell cycle distribution of AGS and NCI-N78 cells was examined using flow cytometry as previously described.21 Briefly, AGS and NCI-N78 cells were treated with danusertib at concentrations of 0.01, 0.1, and 0.5 μM for 24 hours. In separate experiments, AGS and NCI-N78 cells were treated with 0.5 μM danusertib for 4, 8, 12, 24, 48, and 72 hours. After the cells were treated with danusertib, they were trypsinized and fixed in 3 mL of 70% ethanol at −20°C overnight. Following fixation, the cells were collected and resuspended in 1 mL of phosphate-buffered saline containing 1 mg/mL RNase A and 50 μM/mL propidium iodide. The cells were then incubated in the dark for 30 minutes at room temperature. A total number of 1×104 cells were subject to cell cycle analysis using a flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
Quantification of cellular apoptosis
The effect of danusertib on apoptosis of AGS and NCI-N78 cells was quantitated using the Annexin V:PE apoptosis detection kit according to the manufacturer’s instructions. Cells were treated with danusertib at concentrations of 0.01, 0.1, and 0.5 μM for 24 hours and then trypsinized and washed twice with cold phosphate-buffered saline. The cells were then resuspended at a concentration of 1×106/mL cells in 1× binding buffer (BD Biosciences Inc). A quota of cell suspension (100 μL) was transferred into a clean 5 mL tube and incubated with 5 μL of Annexin V:PE and 5 μL of 7-amino-actinomycin D (a vital nucleic acid dye) in the dark for 15 minutes at room temperature. A quota of 1× binding buffer (400 μL) was then added to each tube, and the number of apoptotic cells was quantified using a flow cytometer within one hour of adding the binding buffer. Cells that stain positive for Annexin V:PE and negative for 7-amino-actinomycin D are undergoing apoptosis, cells that stain positive for both Annexin V:PE and 7-amino-actinomycin D are either in the final stage of apoptosis, are undergoing necrosis, or are already dead, and cells that stain negative for both Annexin V:PE and 7-amino-actinomycin D are alive and not undergoing measurable apoptosis.
Determination of cellular autophagy
To determine the effect of danusertib on autophagy in AGS and NCI-N78 cells, the intracellular autophagy level was examined using flow cytometry as previous described.21 Briefly, the cells were treated with fresh medium alone, control vehicle alone (0.05% dimethyl sulfoxide, v/v), or danusertib at 0.01, 0.1, and 0.5 μM for 24 hours. After 24 hours of incubation, the cells were trypsinized and collected with 1× assay buffer containing 5% fetal bovine serum. The cells were resuspended in 250 μL of phenol red-free culture medium (Invitrogen Inc) containing 5% fetal bovine serum, and 250 μL of diluted Cyto-ID Green stain solution was added to each sample and mixed well. The cells were incubated for 30 minutes at 37°C in the dark. Following incubation, the cells were collected by centrifugation at 250× g for 3 minutes and washed with 1× assay buffer. Subsequently, the cells were resuspended in 500 μL of fresh 1× assay buffer containing 5% fetal bovine serum and subject to flow cytometric analysis within one hour of adding they assay buffer. Cells were analyzed using the green (FL1) channel of a flow cytometer.
Confocal fluorescence microscopy
Confocal microscopic analysis was performed to further examine the cellular autophagy level and the mechanisms of danusertib-induced autophagy in AGS and NCI-N78 cells using a Cyto-ID autophagy detection kit. Briefly, AGS and NCI-N78 cells were seeded into an 8-well chamber slide at 30% confluence. The cells were treated with danusertib at 0.01, 0.1, and 0.5 μM for 24 hours. In separate experiments, to investigate the mechanisms for danusertib-induced autophagy, cells were pretreated with 10 μM WM (a PI3K inhibitor and autophagy blocker) and 10 μM SB202190 (a selective inhibitor of p38 MAPK used as an autophagy inducer), and then cotreated with 0.5 μM danusertib for a further 24 hours. After incubation for 24 hours, the cells reached ~60% of confluence and were washed with 1× assay buffer, following by incubation with 100 μL of microscopy dual detection reagent for 30 minutes at 37°C in the dark. After incubation, the cells were washed with 1× assay buffer to remove the detection reagent, and then examined using a TCS SP2 laser scanning confocal microscope (Leica, Wetzlar, Germany) using a standard fluorescein isothiocyanate filter set for imaging the autophagic signal at wavelengths of 405/488 nm.
Western blot analysis
The levels of various cellular proteins related to the cell cycle, apoptosis, and autophagy were determined using Western blotting assays. AGS and NCI-N78 cells were washed with phosphate-buffered saline after 24 hours of treatment with danusertib at 0.01, 0.1, and 0.5 μM, and lysed on ice with lysis buffer (HEPES at pH 7.5, 150 mmol NaCl, 10% glycerol, 1.5 mmol MgCl2, 1% Triton-X 100, 1 mmol ethylenediaminetetraacetic acid at pH 8.0, 10 mmol sodium pyrophosphate, 10 mmol sodium fluoride, phosphatase inhibitor cocktail, and protease inhibitor cocktail) and centrifuged at 3,000× g for 15 minutes at 4°C. The supernatant was collected and the protein concentrations were measured using the Pierce bicinchoninic acid protein assay kit. An equal amount of protein sample (30 μg) was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer and electrophoresed on 7% or 12% SDS-PAGE minigel after thermal denaturation at 95°C for 5 minutes. The proteins were transferred onto a polyvinylidene difluoride membrane at 400 mA for one hour at 4°C. The membranes were blocked with skim milk and probed with the indicated primary antibody overnight at 4°C, and then blotted with appropriate horseradish peroxidase-conjugated secondary anti-mouse or anti-rabbit antibody. Visualization was performed using an enhanced chemiluminescence kit (BioRad Inc, Hercules, CA, USA) and the blots were analyzed using Image Lab 3.0 (BioRad Inc). The protein level was normalized to the matching densitometric value of the internal control, β-actin.
Statistical analysis
The data are presented as the mean ± standard deviation. Comparisons of multiple groups were achieved by one-way analysis of variance followed by Tukey’s multiple comparison procedure. Differences at P<0.05 were considered to be statistically significant. The assays were performed at least three times independently.
Results
Danusertib decreases viability of human gastric cancer AGS and NCI-N78 cells
The MTT assay was performed to determine the effect of danusertib on the viability of AGS and NCI-N78 cells. The results are shown in Figure 1B. Incubation of cells with danusertib at concentrations ranging from 0.01 to 10 μM for 24 hours led to remarkable inhibition of growth of AGS and NCI-N78 cells (Figure 1B). Viability of AGS cells was 63.0%, 57.5%, 51.1%, 60.7%, 45.7%, and 39.5%, and that of NCI-N78 cells was 76.2%, 63.7%, 49.5%, 62.5%, 42.0%, and 48.7% when the cells were treated with danusertib at concentrations of 0.01, 0.1, 0.5, 1, 5, and 10 μM, for 24 hours, respectively. The IC50 values were 1.45 and 2.77 μM for AGS and NCI-N78 cells, respectively. The results show that danusertib had a potent inhibitory effect on growth of AGS and NCI-N78 cells.
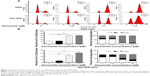
Danusertib induces cell cycle arrest in G2/M phase in AGS and NCI-N78 cells
Having observed the inhibitory effect of danusertib on the viability of AGS and NCI-N78 cells, we next examined the cell cycle distribution of these cells when they were treated with danusertib 0.01, 0.1, and 0.5 μM. The data show that danusertib induces significant cell cycle arrest in the G2/M phase in both cell lines (Figure 2A and B). Treatment of AGS and NCI-N78 cells with danusertib increased the numbers of cells in G2/M phase in a concentration-dependent manner. In comparison with the control cells (29.1%), the percentage of AGS cells arrested in G2/M phase was 80.2% and 83.2% when treated with danusertib 0.1 and 0.5 μM, respectively (P<0.001, Figure 2A and B). Similarly, compared with the control cells (10.5%), the percentage of NCI-N78 cells arrested in G2/M phase was 46.5% and 62.9% when treated with danusertib 0.1 and 0.5 μM, respectively (P<0.001, Figure 2A and B). Treatment with danusertib resulted in a marked decrease in numbers of AGS and NCI-N78 cells in G1 phase. The percentage of AGS cells in G1 phase decreased from 58.3% at the basal level to 15.3% and 10.1% when treated with danusertib 0.1 and 0.5 μM, respectively (Figure 2A and B). The percentage of NCI-N78 cells decreased from 65.6% at the basal level to 37.6% and 20.4% when treated with danusertib 0.1 and 0.5 μM, respectively (Figure 2A and B). However, there was no significant difference in the numbers of cells in S phase in either cell line when treated with danusertib.
Further, we evaluated the effect of treatment with 0.5 μM danusertib on cell cycle distribution lines over 72 hours in both cell lines. The results show a marked increase in the number of cells in G2/M phase but a decrease in the number of cells in G1 phase (Figure 3A and B). In comparison with the control, the percentage of AGS cells in G2/M phase was increased from 13.8% at the basal level to 30.4%, 44.2%, 43.2%, 80%, 92.8%, and 94.8% on treatment with 0.5 μM danusertib for 4, 8, 12, 24, 48, and 72 hours, respectively (P<0.001, Figure 3A and B). Similarly, the percentage of NCI-N78 cells in G2/M phase increased from 15.4% at the basal level to 28%, 39.5%, 68.0%, 79.0%, and 77.7% when the cells were incubated with 0.5 μM danusertib for 8, 12, 24, 48, and 72 hours, respectively (P<0.001, Figure 3A and B). In contrast, the percentage of AGS cells in G1 phase was decreased from 53.4% at the basal level to 38.6%, 25.6%, 22.0%, 9.9%, 2.4%, and 1.5% on exposure to 0.5 μM danusertib for 4, 8, 12, 24, 48, and 72 hours, respectively (Figure 3A and B). For NCI-N78 cells, the percentage of cells in G1 phase decreased from 73.5% at the basal level to 68.6%, 59.1%, 45.2%, 22.6%, 15.1%, and 15.3% after incubation with 0.5 μM danusertib for 4, 8, 12, 24, 48, and 72 hours, respectively (Figure 3A and B). Taken together, these results show that danusertib induces cell cycle arrest in G2/M phase in human gastric cancer AGS and NCI-N78 cells in a time-dependent and concentration-dependent manner.
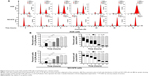
Danusertib increases expression of p53, p21 Waf1/Cip1, and p27 Kip1 and decreases expression of cyclin B1 and CDC2 in AGS and NCI-N78 cells
After finding that danusertib had a marked inducing effect on cell cycle arrest in G2/M phase in AGS and NCI-N78 cells, we further examined the expression level of key regulators responsible for G2 to M phase checkpoint using the Western blotting assay to explore the possible mechanisms for danusertib-induced cell cycle arrest in both cell lines. As shown in Figure 4A and B, treatment with danusertib significantly inhibited expression of positive regulators but enhanced the negative regulators responsible for the G2 to M phase transition. Incubating AGS cells with danusertib 0.1 and 0.5 μM for 24 hours led to a 31% and 46% decrease in CDC2 expression and to a 51% and 53% reduction in cyclin B1 expression, respectively (Figure 4A and B). Similarly, treating NCI-N78 cells with 0.5 μM danusertib resulted in a 45% decrease in expression of CDC2. There was also a 75% and 70% reduction in expression of cyclin B1 when NCI-N78 cells were treated with danusertib 0.1 and 0.5 μM for 24 hours, respectively (Figure 4A and B). These results indicate that downregulation of CDC2 and cyclin B1 expression contributes, at least in part, to the inhibitory effect of danusertib on cell growth in AGS and NCI-N78 cells.
p21 Waf1/Cip1 and p27 Kip1 act as inhibitors of cell cycle progression by inhibiting the activity of cyclin-dependent kinases in association with CDK/cyclin complexes.22,23 p53 is a tumor suppressor protein that plays a major role in the cellular response to DNA damage and other genomic aberrations,24 and activation of p53 can lead to either cell cycle arrest and DNA repair or apoptosis. Therefore, to further investigate the possible mechanisms for the cell cycle arrest-inducing effect of danusertib in AGS and NCI-N78 cells, p21 Waf1/Cip1, p27 Kip1, and p53 expression levels were evaluated in both cell lines when treated with danusertib 0.01, 0.1, and 0.5 μM for 24 hours (Figure 4A and B). In comparison with the control, the expression levels were increased 2.2-fold and 2.7-fold for p21 Waf1/Cip1, 1.4-fold and 1.5-fold for p27 Kip1, and 3.3-fold and 3.5-fold for p53 in AGS cells when treated with danusertib 0.1 and 0.5 μM for 24 hours, respectively (Figure 4A and B). In NCI-N78 cells, treatment with danusertib for 24 hours had a similar inducing effect on expression of p21 Waf1/Cip1, p27 Kip1, and p53. Expression levels were increased by 1.4-fold and 1.5-fold for p21 Waf1/Cip1 and by 1.5-fold and 1.7-fold for p27 Kip1 when cells were incubated with danusertib 0.1 and 0.5 μM for 24 hours, respectively (Figure 4A and B). Moreover, treating NCI-N78 cells with 0.5 μM danusertib increased expression of p53 by 1.6-fold (Figure 4A and B). Taken together, these results show that danusertib can upregulate expression of p21 Waf1/Cip1, p27 Kip1, and p53, contributing to the inducing effect of danusertib on cell cycle arrest in AGS and NCI-N78 cells.
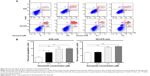
Danusertib induces mitochondria-dependent apoptosis in AGS and NCI-N78 cells
To explore the role of danusertib in the treatment of gastric cancer further, we examined the effect of danusertib on apoptosis in AGS and NCI-N78 cells using flow cytometry. The results are shown in Figure 5. When AGS and NCI-N78 cells were incubated with danusertib 0.01, 0.1, and 0.5 μM for 24 hours, there was a concentration-dependent increase in apoptosis in both cell lines (Figure 5A and B). Treating AGS cells with danusertib 0.01, 0.1, and 0.5 μM for 24 hours increased the total percentage of apoptotic cells (early and late apoptosis) from 3.7% at the basal level to 7.2%, 9.7%, and 10.1%, and there was a 1.9-fold, 2.6-fold, and 2.7-fold increase in apoptotic AGS cells compared with the control, respectively (P<0.05 or P<0.01, Figure 5A and B). Incubation of NCI-N78 cells with danusertib 0.01, 0.1, and 0.5 μM for 24 hours increased the total proportion of apoptotic cells from 5.1% at the basal level to 7.5%, 11%, and 11.7%, respectively. In comparison with the control, there was a 1.5-fold and 1.6-fold increase in the proportion of apoptotic NCI-N78 cells in response to treatment with danusertib 0.1 and 0.5 μM, respectively (P<0.01 or P<0.001, Figure 5A and B). The results clearly show that danusertib induces apoptotic cell death in AGS and NCI-N78 cells.
In order to explore the possible mechanisms for the apoptosis-inducing effect of danusertib in AGS and NCI-N78 cells, we first tested the effect of danusertib on expression of the proapoptotic protein Bax and the antiapoptotic proteins Bcl-2 and Bcl-xl in these two cell lines. As shown in Figure 6, treatment with danusertib markedly increased the expression of Bax, while reducing the expression of Bcl-xl and Bcl-2 in both cell lines. Incubating AGS and NCI-N78 cells with danusertib 0.5 μM for 24 hours resulted in a 1.3-fold and 1.4-fold increase in the expression of Bax, respectively (P<0.05, Figure 6A and B). In contrast, expression of Bcl-xl and Bcl-2 decreased by 43.9% and 57.7% when AGS cells were treated with danusertib 0.5 and 0.1 μM for 24 hours, respectively (P<0.05, Figure 6A and B). In NCI-N78 cells, expression of Bcl-xl decreased by 26.8% when cells were treated with 0.5 μM danusertib; expression of Bcl-2 was reduced by 28% and 31% when cells were treated with danusertib 0.1 and 0.5 μM, respectively (P<0.05, Figure 6A and B). Moreover, given the negative regulatory effect of PUMA on antiapoptotic proteins, the effect of danusertib on expression of PUMA was also examined. Incubation of AGS and NCI-N78 cells with 0.5 μM danusertib increased the expression of PUMA by 1.7-fold and 1.5-fold (P<0.05, Figure 6A and B).
The effect of danusertib on the mitochondria-related apoptotic pathway was examined further in AGS and NCI-N78 cells. Release of cytochrome c from the mitochondria to the cytosol is the early event and initiates the caspase-dependent apoptotic cascade, with caspase cascade been activated.25 As such, we examined the effect of treatment with danusertib on release of cytochrome c from the mitochondria to the cytosol in AGS and NCI-N78 cells. Treatment of these cells with danusertib for 24 hours significantly increased the amount of cytosolic cytochrome c and increased the levels of c-caspase 9 and c-caspase 3 (Figure 6A and B). Taken together, these results indicate that danusertib induces mitochondria-dependent apoptosis in AGS and NCI-N78 cells.
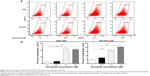
Danusertib induces autophagy in AGS and NCI-N78 cells via inhibition of the PI3K/Akt/mTOR signaling pathway
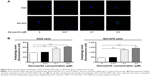
After observing danusertib-induced apoptosis in AGS and NCI-N78 cells, we next examined the effect of danusertib on type II programmed cell death, ie, autophagy, in these cell lines. Flow cytometry and confocal fluorescence microscopy were performed to determine the level of autophagy in AGS and NCI-N78 cells. As shown in Figure 7, incubation of AGS and NCI-N78 cells with danusertib for 24 hours increased the percentage of autophagic cells in a concentration-dependent manner. The percentage of autophagic cells at the basal level was 2.7% and 4.7% for AGS and NCI-N78 cells, respectively. Compared with the control, treating AGS cells with 0.1 and 0.5 μM danusertib resulted in a 7.9-fold and 7.8-fold increase in the percentage of autophagic cells, respectively (P<0.001, Figure 7A and B). Similarly, treatment of NCI-N78 cells with danusertib 0.1 and 0.5 μM for 24 hours led to a 3.4-fold and 4.4-fold increase in the percentage of autophagic cells, respectively (Figure 7A and B). We further confirmed the autophagy-inducing effect of danusertib in AGS and NCI-N78 cells using confocal microscopy. The results indicate that treatment with danusertib significantly increased the autophagy level in a concentration-dependent manner in AGS and NCI-N78 cells (Figure 8A and B). There was a 1.4-fold and 1.6-fold increase in autophagy of AGS cells in response to treatment with danusertib 0.1 and 0.5 μM for 24 hours, respectively, when compared with the control (Figure 8A and B). Similarly, in comparison with the control, there was a 2.8-fold and 3.1-fold increase in autophagy when NCI-N78 cells were treated with danusertib 0.1 and 0.5 μM for 24 hours, respectively (Figure 8A and B). These results indicate that danusertib induces autophagy in both AGS and NCI-N78 cells.
Next, we explored the possible mechanisms for the autophagy-inducing effect of danusertib in AGS and NCI-N78 cells. It has been reported that PI3K/Akt/mTOR signaling plays a critical role in regulation of autophagy, and targeting PI3K/Akt/mTOR signaling has been proposed to be a promising strategy for cancer therapy.26–28 Therefore, we initially examined phosphorylation of PI3K at Tyr458, AMPK at Thr172, and p38 MAPK at Thr180/Tyr182, which are upstream signaling molecules regulating the Akt/mTOR pathway and have an important role in regulation of cell survival and cell death.29,30 Treatment of the cells with danusertib significantly inhibited phosphorylation of PI3K at Tyr458, but did not significantly affect expression of total phosphatidylinositol-4 5-bisphosphate 3-kinase when compared with the control. The ratio of p-PI3K over PI3K was decreased in AGS and NCI-N78 cells in a concentration-dependent manner in response to treatment with danusertib (Figure 9A and B, respectively). There was a 45.0% and 52.0% decrease in the p-PI3K/PI3K ratio when AGS cells were treated with danusertib 0.1 and 0.5 μM for 24 hours compared with the control, respectively (P<0.01; Figure 9A and B). In NCI-N78 cells, the ratio of p-PI3K over PI3K was decreased by 34% and 38% in response to 24 hours of treatment with danusertib 0.1 and 0.5 μM, respectively, when compared with the control (P<0.05; Figure 9B).
Phosphorylation at Thr172 is required for activation of AMPK, which has a promoting effect on autophagy.31 Under certain circumstances, AMPK promotes autophagy by directly activating Unc-51-like kinase 1 via phosphorylation of Ser317 and Ser777.32 As shown in Figure 9A and B, danusertib had a promoting effect on phosphorylation of AMPK at Thr172 in both cell lines, whereas there was no significant change in expression of total AMPK when compared with the control cells. Therefore, with increasing concentrations of danusertib, an increased ratio of p-AMPK over AMPK was observed in AGS and NCI-N78 cells. In AGS cells, there was a 1.6-fold increase in the p-AMPK/AMPK ratio in response to treatment with 0.5 μM danusertib for 24 hours (P<0.05, Figure 9A and B). Similarly, treatment of NCI-N78 cells with 0.5 μM danusertib was associated with a 1.7-fold increase in the ratio of p-AMPK over AMPK (P<0.05, Figure 9A and B).
p38 MAPK has an important role in the regulation of cellular responses to various stimuli.33 In contrast with the promoting effects with regard to phosphorylation of AMPK, danusertib inhibited the activation of p38 MAPK at Thr180/Tyr182 in both AGS and NCI-N78 cells (Figure 9A and B). In AGS cells, there was a concentration-dependent inhibition of phosphorylation of p38 MAPK at Thr180/Tyr182, without significant changes in expression of total p38 MAPK, when compared with the control cells. The ratio of p-p38 MAPK over p38 MAPK was decreased by 36% and 37% in response to treatment with danusertib 0.1 and 0.5 μM for 24 hours, respectively (P<0.05, Figure 9A and B). In comparison with the control cells, incubation of NCI-N78 cells with danusertib 0.1 and 0.5 μM resulted in a 45% and 37% decrease in the p-p38 MAPK/p38 MAPK ratio (P<0.05, Figure 9A and B). These findings indicate that inhibition of phosphorylation of PI3K and p38 MAPK but promotion of phosphorylation of AMPK might contribute to the autophagy-inducing effect of danusertib in AGS and NCI-N78 cells.
Having determined the regulatory effects of danusertib on PI3K, AMPK, and p38 MAPK, we next evaluated the effect of danusertib on phosphorylation of Akt at Ser473 and mTOR at Ser2448 and on expression of PTEN in AGS and NCI-N78 cells (Figure 9A). As a downstream effector of PI3K, Akt is involved in regulation of various signaling pathways involved in cell metabolism, proliferation, survival, growth, and angiogenesis.34 Akt can activate mTOR at Ser2448, which plays a key role in cell growth, autophagic cell death, and homeostasis.35 PTEN is a negative regulator of PI3K and inhibits Akt/mTOR and MAPK signaling, leading to inhibition of cell growth and cell death.36 The results show that danusertib inhibited activation of Akt at Ser473 without a significant change in expression of total Akt in either cell line (Figure 9A and B). The ratio of p-Akt over Akt was decreased by 34.0% and 31.0% when AGS cells were treated with danusertib 0.1 and 0.5 μM for 24 hours, compared with control cells. Similarly, the ratio of p-Akt over Akt was decreased by 55.0% and 62.0% in NCI-N78 cells treated with danusertib 0.1 and 0.5 μM, respectively (Figure 9A and B). Expression of PTEN was significantly increased in AGS and NCI-N78 cells treated with danusertib (Figure 9). In addition, exposure of these cells to danusertib resulted in a marked decrease in phosphorylation of mTOR at Ser2448, whereas there was no significant change in expression of total mTOR in these two cell lines. The ratio of p-mTOR over mTOR decreased by 42.0% when AGS cells were treated with danusertib 0.5 μM for 24 hours (Figure 9A and B). In NCI-N78 cells, the p-mTOR/mTOR ratio decreased by 27.0% and 32.0% in response to treatment with danusertib 0.1 and 0.5 μM for 24 hours, respectively (Figure 9A and B).
Beclin 1 and LC3 are two important markers of vesicle expansion and formation during the autophagy process.37–39 Therefore, we evaluated the effect of danusertib on expression of beclin 1, LC3-I, and LC3-II in AGS and NCI-N78 cells. Treating the cells with danusertib significantly increased the expression of beclin 1 and conversion of LC3-I to LC3-II in AGS and NCI-N78 cells (Figure 9). There was a 1.4-fold and 1.4-fold increase in expression of beclin 1 in AGS cells treated with 0.1 and 0.5 μM danusertib for 24 hours (P<0.05, Figure 9A and B). In NCI-N78 cells, there was a 1.3-fold, 1.5-fold, and 1.5-fold increase in expression of beclin 1 in response to treatment with 0.01, 0.1, and 0.5 μM danusertib for 24 hours, respectively (P<0.05 or P<0.01, Figure 9A and B). In addition, there was a marked increase in expression of LC3-II in both AGS and NCI-N78 cells (Figure 9A and B). Compared with control cells, there was a 1.3-fold increase in the ratio of LC3-II/LC3-I when AGS cells were treated with danusertib 0.5 μM for 24 hours (P<0.05 or P<0.01, Figure 9A and B). In NCI-N78 cells, treatment with danusertib 0.1 and 0.5 μM resulted in a 3.6-fold and 3.5-fold increase in the ratio of LC3-II/LC3-I, respectively (P<0.001, Figure 9A and B). These results indicate that danusertib-induced autophagy in AGS and NCI-N78 cells is, at least in part, ascribed to inhibition of the PI3K/Akt/mTOR signaling pathway.
SB202190 and WM enhance danusertib-induced autophagy in AGS and NCI-N78 cells
Our data indicate danusertib-induced autophagy involving the PI3K/Akt/MTOR signaling pathway. To confirm the mechanism for the proautophagic effect of danusertib in AGS and NCI-N78 cells, we used SB202190 and WM and examined the autophagy level using flow cytometry and confocal microcopy. SB202190 is a p38 MAPK-selective inhibitor that can induce formation of autophagic vacuoles through cross-inhibition of the PI3K/mTOR pathway.40 WM is a well-known PI3K inhibitor. Incubation of SB202190 and WM at 10 μM significantly enhanced danusertib-induced autophagy when compared with the control cells treated with danusertib alone (Figure 10A and B). In AGS cells, both SB202190 and WM at 10 μM significantly increased danusertib-induced autophagy by 41.8% and 20.2%, respectively, when compared with the control cells treated with danusertib alone (Figure 10A and B). Similarly, in NCI-N78 cells, SB202190 and WM at 10 μM significantly increased danusertib-induced autophagy by 41.2% and 14.5%, respectively, compared with the control cells treated with danusertib alone (Figure 10A and B). Likewise, the confocal microscopy results showed that SB202190 and WM at 10 μM significantly enhanced danusertib-induced autophagy compared with the control cells treated with danusertib alone (Figure 11A and B). Taken together, the results suggest danusertib-induced autophagy in AGS and NCI0N78 cells involving signaling pathways mediated by PI3K and p38 MAPK.
Danusertib inhibits the EMT phenotype in AGS and NCI-N78 cells
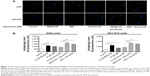
We also examined the effect of danusertib on EMT in AGS and NCI-N78 cells. It has been well recognized that EMT is a critical process involved in the initiation, growth, invasion, and metastasis of cancer, and that inhibition of EMT can result in tumor regression.41 Reduction in expression of cell adhesion molecules drives EMT with the involvement of a number of key functional proteins, such as E/N-cadherin, snail, slug, vimentin, claudin 1, and β-catenin.42 Herein, we examined the effect of treatment with danusertib on EMT-associated markers in AGS and NCI-N78 cells using Western blotting assays. As shown in Figure 12A and B, incubation of AGS cells with danusertib resulted in a marked increase in expression of E-cadherin and a decrease in expression of N-cadherin. In comparison with the control cells, there was a 1.3-fold and 1.5-fold increase in expression of E-cadherin and a 47.0% and 52.0% decrease in expression of N-cadherin, when AGS cells were treated with danusertib 0.1 and 0.5 μM, respectively (P<0.05 or P<0.01, Figure 12A and B). In NCI-N78 cells, there was a 1.5-fold increase in expression of E-cadherin in response to treatment with 0.5 μM danusertib (P<0.05, Figure 12A and B). Meanwhile, expression of N-cadherin was decreased by 39% and 37% when NCI-N78 cells were treated with danusertib 0.1 and 0.5 μM (P<0.01, Figure 12A and B).
We next examined the expression of snail and slug, which are suppressors of E-cadherin in EMT.42 As shown in Figure 12, danusertib significant reduced the expression of snail and slug in both cell lines (Figure 12). In AGS cells, treatment with danusertib 0.1 and 0.5 μM resulted in a 76% and 70% reduction in expression of snail, respectively, compared with control cells (P<0.01, Figure 12A and B), and incubation of cells with 0.5 μM danusertib caused a 62% decrease in expression of slug when compared with control cells (P<0.05, Figure 12A and B). Similarly, in comparison with the control cells, there was a 28% and 27% decrease in expression of snail when NCI-N78 cells were treated with 0.1 and 0.5 μM danusertib, and there was a 42% decrease in expression of slug when NCI-N78 cells were treated with 0.5 μM danusertib (Figure 12A and B).
Vimentin is a type III intermediate filament protein expressed in mesenchymal cells,42 and β-catenin can serve as an integral component of a protein complex in adherens junctions involved in regulation of EMT.42 Treatment of cells with danusertib 0.1 and 0.5 μM decreased expression of vimentin in NCI-N78 cells by 50% and 44%, respectively (P<0.05; Figure 12B), but did not significantly alter expression of vimentin in AGS cells (P=0.078; Figure 12A). There was a significant 41% and 43% reduction in expression of β-catenin in AGS cells treated with danusertib 0.1 and 0.5 μM, respectively, for 24 hours. However, we did not observe a significant reduction in expression of β-catenin in NCI-N78 cells. Moreover, treatment with danusertib 0.5 μM significantly increased the expression of claudin 1 in NCI-N78 cells, but not in AGS cells. Taken together, the results show an inhibitory effect of danusertib on EMT in AGS and NCI-N78 cells.
Discussion
Gastric cancer often responds poorly to current therapies, with an unfavorable clinical outcome that requires novel therapies for an improved therapeutic outcome. The Aurora kinases have been proposed to be promising therapeutic targets in the treatment of cancer.43 Recently, a number of small molecules have been developed as Aurora kinase inhibitors.44 Danusertib is a pan-Aurora kinase inhibitor exhibiting a potent effect in various types of cancer. In the present study, we observed a potent inhibitory effect of danusertib on cell proliferation in two gastric cancer cell lines, ie, AGS and NCI-N78. We found that danusertib induces cell cycle arrest and promotes cell apoptosis and autophagy, with involvement of signaling pathways mediated by PI3K/Akt/mTOR, p38 MAPK, and AMPK in AGS and NCI-N78 cell lines. In addition, danusertib inhibits the EMT-like phenotype in both cell lines.
The Aurora family consist of AURKA/B/C with different cellular localizations and functions.7,45 They have important roles in the regulation of chromosome assembly and segregation during mitosis.7 As key cell cycle regulators, the Aurora kinases regulate G2/M transition. Inhibition of the activity and/or expression of Aurora kinases leads to G2/M arrest and apoptosis, whereas ectopic expression allows cells to bypass the G2/M DNA damage checkpoint.46 Abnormalities in the activity and expression of Aurora kinases have been closely associated with the initiation, development, and progression of a variety of human malignancies, including gastric cancer.12,14,47,48 Recently, danusertib, a small ATP-competitive molecule that inhibits AURKA/B/C, has entered Phase II clinical trials for patients with solid tumors.20 Danusertib has a strong inhibitory effect on AURKA, as determined by inhibition of autophosphorylation at Thr288, and also on AURKB, as determined by inhibition of its substrate histone H3 at position Ser10.49,50
In the present study, we found that danusertib arrested AGS and NCI-N78 cells during G2/M phase in a concentration-dependent manner. The CDC2-cyclin B1 complex is pivotal in the regulation of G2/M phase transition and mitosis. We observed a significant decrease in expression of cyclin B1 and CDC2 in AGS and NCI-N78 cells treated with danusertib, which explains the inducing effect of danusertib on G2/M phase arrest in both cell lines. It has been reported that p21 Waf1/Cip1, a cyclin-dependent kinase inhibitor regulated by p53, can bind to the CDK1/CDC2-cyclin B1 complex, thereby inducing cell cycle arrest.22,23 The findings of the present study show that expression of p53, p27 Kip1, and p21 Waf1/Cip1 was increased in AGS and NCI-N78 cells treated with danusertib, which probably contributes to the inhibitory effect of danusertib on cell proliferation and inducing effect on cell cycle arrest. The results indicate that upregulation of p53, p21 Waf1/Cip1, and p27 Kip1 expression by danusertib may result in arrest of G2 phase in AGS and NCI-N78 cell lines.
Accumulating evidence shows that manipulating apoptosis by modulation of the key regulators of apoptosis is a promising therapeutic strategy in the treatment of cancer.51,52 It has been shown that apoptosis can be activated by two main pathways, ie, the extrinsic death receptor pathway and the intrinsic mitochondria/cytochrome c-mediated pathway activating the caspase family.25,53 In the present study, we observed a concentration-dependent proapoptotic effect of danusertib on AGS and NCI-N78 cells. It has been shown that disruption of the mitochondria and subsequent release of cytochrome c initiate the process of apoptosis, which is triggered by proapoptotic members of the Bcl-2 family but antagonized by antiapoptotic members of this family.25 In the present study, the increase in cytosolic cytochrome c may be attributed to the increased expression of Bax and decreased expression of Bcl-xl and Bcl-2 when AGS and NCI-N78 cells were treated with danusertib. Further, it has been demonstrated that antiapoptotic members of the Bcl-2 family can be negatively regulated by post-translational modification and/or by increased expression of PUMA, an essential regulator of p53-mediated cell apoptosis.25 It has also been reported that p53 plays an important role in AURKB inhibitor-mediated apoptosis.54 In addition, cytosolic cytochrome c triggers the caspase cascade.25 In the present study, our findings suggest that the increased cytosolic level of cytochrome c subsequently activates caspase 9. Activated caspase 9 in turn activates caspase 3, which ultimately induces apoptosis via a decrease in the Bcl-2 level. These results indicate that danusertib induces mitochondria-dependent apoptosis in both AGS and NCI-N78 cells, with involvement of p53.
The role of autophagy in the survival and death of cancer cells is controversial.55 Autophagy is an important type of programmed cell death involving the engulfment and degradation of nonessential or abnormal cellular organelles and proteins in living cells.29,30 Autophagy has two primary and opposing functions, including cytoprotective and cytotoxic effects,55–57 which renders the role of autophagy unclear in the treatment of cancer. The PI3K/Akt/mTOR signaling pathway is central to the regulation of cell growth, motility, protein synthesis, survival, and metabolism in response to a variety of stimuli.25,53,58 PI3K activates the serine/threonine kinase Akt, which in turn leads to the phosphorylation and activation of the serine/threonine kinase mTOR through a cascade of regulators.58 In the present study, danusertib induced autophagy in both AGS and NCI-N78 cells, and this may contribute to its anticancer effect. Inhibition of the PI3K/Akt/mTOR signaling pathway contributes to the autophagy-inducing effect of danusertib in AGS and NCI-N78 cells. The role of autophagy in cancer treatment is multifarious, which can function as a tumor suppressor at early stage of tumor development but can also be used by cancer cells as a cytoprotection mechanism to promote survival in established tumors, thus, it needs to be further elucidated.29,30
AMPK and p38 MAPK are upstream regulators of Akt/mTOR. It has been demonstrated that AMPK is an important regulator of cell death via activation of Janus kinase and p53 and via inhibition of mTOR.30 In our study, there was significant activation of AMPK in response to treatment with danusertib, which may contribute to inhibition of mTOR. Further, p38 MAPK is responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and regulates cell differentiation, apoptosis, and autophagy.25,30 We found that danusertib significantly suppressed the phosphorylation of p38 MAPK. Notably, SB202190, a p38 MAPK inhibitor, markedly increased the degree of apoptotic and autophagic cell death. Taken together, both AMPK and p38 MAPK play important roles in danusertib-induced autophagy in gastric cancer cells.
In addition, our findings show that danusertib significantly suppressed EMT in AGS and NCI-N78 cells. EMT, which is characterized by a gain in mesenchymal cell markers and a loss of epithelial markers,59 plays an important role in progression, metastasis, and drug resistance in various solid tumors, including cutaneous, prostatic, mammary, hepatic, gastric, pancreatic, and colorectal cancers.60,61 It has been reported that AURKA promotes EMT in breast cancer.62 Thus, an Aurora kinase inhibitor should be able to suppress EMT. The findings of our present study show that danusertib significantly increases the expression of E-cadherin but decreases the expression of N-cadherin. Previously, it has been reported that EMT-inducing transcription factors, including Twist, snail, slug, Zeb-1, and Zeb-2, induce dramatic spreading and morphological changes in gastric cancer cells via suppression of E-cadherin and induction of mesenchymal markers.63,64 In agreement with a previous study, we found that treatment with danusertib inhibited the expression of snail and slug in AGS and NCI-N78 cells. Notably, danusertib significantly increased the expression of claudin 1 but decreased the expression of vimentin only in NCI-N78 cells, and significantly inhibited expression of β-catenin only in AGS cells. This may be due to differences in the origin of these cell lines or to cytogenetic and other related factors.
In conclusion, danusertib inhibited cell proliferation, activated the mitochondria-dependent apoptotic pathway, and induced autophagy in human AGS and NCI-N7 cells. Inhibition of PI3K/Akt/mTOR and p38 MAPK along with activation of AMPK signaling pathways is involved in the autophagy-inducing effect of danusertib in AGS and NCI-N7 cells. In addition, danusertib suppressed EMT, contributing to its anticancer effect. Collectively, danusertib may represent a new anticancer drug that kills gastric cancer cells. More studies are needed to reveal the underlying mechanisms involved and other potential targets and to verify the efficacy and safety of danusertib in the treatment of gastric cancer.
Acknowledgments
The authors appreciate the financial support of the Startup Fund of the College of Pharmacy, University of South Florida, Tampa, FL, USA. Z-WZ holds a postdoctoral scholarship from the College of Pharmacy, University of South Florida.
Disclosure
The authors report no conflicts of interest in this work.
References
Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–260. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2(2):77–84. | ||
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed October 28, 2014. | ||
Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD, USA: National Cancer Institute; 2013. Available from: http://seer.cancer.gov/csr/1975_2011/. Accessed October 28, 2014. | ||
US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta, GA, USA: Centers for Disease Control and Prevention; 2013. Available from: http://apps.nccd.cdc.gov/uscs/. Accessed October 28, 2014. | ||
Bolanos-Garcia VM. Aurora kinases. Int J Biochem Cell Biol. 2005;37(8):1572–1577. | ||
Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in Aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. | ||
Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the Auroras come into view. Curr Opin Cell Biol. 2003;15(6):672–683. | ||
Gontarewicz A, Brummendorf TH. Danusertib (formerly PHA-739358) – a novel combined pan-Aurora kinases and third generation Bcr-Abl tyrosine kinase inhibitor. Recent Results Cancer Res. 2010;184:199–214. | ||
Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22(4):451–464. | ||
Wang J, Yang S, Zhang H, et al. Aurora-A as an independent molecular prognostic marker in gastric cancer. Oncol Rep. 2011;26(1):23–32. | ||
Tarnawski A, Pai R, Chiou SK, Chai J, Chu EC. Rebamipide inhibits gastric cancer growth by targeting survivin and Aurora-B. Biochem Biophys Res Commun. 2005;334(1):207–212. | ||
Wang W, Zhang N, Wang J, Bu XM, Zhao CH. Inhibition of proliferation, viability, migration and invasion of gastric cancer cells by Aurora-A deletion. Asian Pac J Cancer Prev. 2011;12(10):2717–2720. | ||
Katsha A, Soutto M, Sehdev V, et al. Aurora kinase A promotes inflammation and tumorigenesis in mice and human gastric neoplasia. Gastroenterology. 2013;145(6):1312–1322. | ||
Katsha A, Arras J, Soutto M, Belkhiri A, El-Rifai W. AURKA regulates JAK2-STAT3 activity in human gastric and esophageal cancers. Mol Oncol. June 3, 2014. [Epub ahead of print]. | ||
Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and Aurora kinases in cancer. Nat Rev Cancer. 2010;10(12):825–841. | ||
Gontarewicz A, Balabanov S, Keller G, et al. Simultaneous targeting of Aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008;111(8):4355–4364. | ||
Carpinelli P, Ceruti R, Giorgini ML, et al. PHA-739358, a potent inhibitor of Aurora kinases with a selective target inhibition profile relevant to cancer. Mol Cancer Ther. 2007;6(12 Pt 1):3158–3168. | ||
Meulenbeld HJ, Mathijssen RH, Verweij J, de Wit R, de Jonge MJ. Danusertib, an Aurora kinase inhibitor. Expert Opin Investig Drugs. 2012;21(3):383–393. | ||
Li YC, He SM, He ZX, et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014;344(2):239–259. | ||
Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol. 2013;25(1):52–58. | ||
Yoon MK, Mitrea DM, Ou L, Kriwacki RW. Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem Soc Trans. 2012;40(5):981–988. | ||
Carvajal LA, Manfredi JJ. Another fork in the road – life or death decisions by the tumour suppressor p53. EMBO Rep. 2013;14(5):414–421. | ||
Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. | ||
Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16(8):797–803. | ||
Maira SM, Furet P, Stauffer F. Discovery of novel anticancer therapeutics targeting the PI3K/Akt/mTOR pathway. Future Med Chem. 2009;1(1):137–155. | ||
Wu P, Hu YZ. PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress. Curr Med Chem. 2010;17(35):4326–4341. | ||
Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. | ||
Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19(1):87–95. | ||
Dunlop EA, Tee AR. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem Soc Trans. 2013;41(4):939–943. | ||
Kim J, Guan KL. Regulation of the autophagy initiating kinase ULK1 by nutrients: roles of mTORC1 and AMPK. Cell Cycle. 2011;10(9):1337–1338. | ||
Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692. | ||
Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. | ||
Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. | ||
Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet. 2013;163C(2):114–121. | ||
Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. | ||
Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the beclin 1 interactome. EMBO J. 2010;29(3):515–516. | ||
Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. | ||
Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. | ||
Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12(12):1383–1430. | ||
Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol. 2011;8(8):428–439. | ||
Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14(6):1639–1648. | ||
Lok W, Klein RQ, Saif MW. Aurora kinase inhibitors as anti-cancer therapy. Anticancer Drugs. 2010;21(4):339–350. | ||
Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4(11):842–854. | ||
Hilton JF, Shapiro GI. Aurora kinase inhibition as an anticancer strategy. J Clin Oncol. 2014;32(1):57–59. | ||
Dar AA, Belkhiri A, El-Rifai W. The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene. 2009;28(6):866–875. | ||
Chiou SK, Hoa N, Hodges A, Ge L, Jadus MR. Indomethacin promotes apoptosis in gastric cancer cells through concomitant degradation of survivin and Aurora B kinase proteins. Apoptosis. 2014;19(9):1378–1388. | ||
Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003;12(4):851–862. | ||
Crosio C, Fimia GM, Loury R, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22(3):874–885. | ||
Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–194. | ||
Fernandez-Luna JL. Apoptosis regulators as targets for cancer therapy. Clin Transl Oncol. 2007;9(9):555–562. | ||
Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–183. | ||
Ikezoe T, Yang J, Nishioka C, Yokoyama A. p53 is critical for the Aurora B kinase inhibitor-mediated apoptosis in acute myelogenous leukemia cells. Int J Hematol. 2010;91(1):69–77. | ||
Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74(3):647–651. | ||
Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2(2):85–90. | ||
Yang ZJ, Chee CE, Huang S, Sinicrope F. Autophagy modulation for cancer therapy. Cancer Biol Ther. 2011;11(2):169–176. | ||
Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10(3):143–153. | ||
Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118 Pt 19:4325–4326. | ||
Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66(9):4549–4552. | ||
Kim MC, Cui FJ, Kim Y. Hydrogen peroxide promotes epithelial to mesenchymal transition and stemness in human malignant mesothelioma cells. Asian Pac J Cancer Prev. 2013;14(6):3625–3630. | ||
D’Assoro AB, Liu T, Quatraro C, et al. The mitotic kinase Aurora – a promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERalpha(+) breast cancer cells. Oncogene. 2014;33(5):599–610. | ||
Jung HY, Jung JS, Whang YM, Kim YH. RASSF1A suppresses cell migration through inactivation of HDAC6 and increase of acetylated alpha-tubulin. Cancer Res Treat. 2013;45(2):134–144. | ||
Jia B, Liu H, Kong Q, Li B. Overexpression of ZEB1 associated with metastasis and invasion in patients with gastric carcinoma. Mol Cell Biochem. 2012;366(1–2):223–229. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.