Back to Journals » Clinical Ophthalmology » Volume 8
Dacryocystorhinostomy ostium: parameters to evaluate and DCR ostium scoring
Authors Ali MJ , Psaltis A, Wormald P
Received 9 September 2014
Accepted for publication 25 September 2014
Published 9 December 2014 Volume 2014:8 Pages 2491—2499
DOI https://doi.org/10.2147/OPTH.S73998
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohammad Javed Ali,1 Alkis James Psaltis,2 Peter John Wormald2
1Dacryology Service, L V Prasad Eye Institute, Hyderabad, Telangana, India; 2Department of Surgery–Otorhinolaryngology, Head and Neck Surgery, The University of Adelaide, Adelaide, SA, Australia
Aim: This study aims to provide a systematic protocol for the evaluation of a dacryocystorhinostomy (DCR) ostium and to propose a scoring system to standardize the assessment.
Methods: Retrospective evaluation of 125 consecutive lacrimal ostia post-DCR was performed. Medical records were screened, and photographs and videos were assessed to note the details of various ostial parameters. The major time points in evaluation were 4 weeks, 6 weeks, 3 months, and 6 months post-DCR. The ostia were defined and parameters like shape, size, location, and evolution of ostium were noted. Evaluation parameters were defined for internal common opening (ICO), ostium stents, and ostium granulomas. Ostium cicatrix and synechiae were graded based on their significance. Surgical success rates were computed and ostium characteristics in failed cases were studied.
Results: A total of 125 ostia were evaluated on the aforementioned ostium parameters. Because five ostia showed a complete cicatricial closure with no recognizable features, the remaining 120 ostia were studied. The ostium location was anterior to the axilla of middle turbinate in 85.8% (103/120) of the cases. Moreover, 76.6% (92/120) of the ostia were circular to oval in shape, with a shallow base. The ostium size was >8×5 mm in 78.3% (94/120) of the cases. The ICO was found to be located in the central or paracentral basal area in 75.8% (91/120). The anatomical and functional success rates achieved were 96% and 93.6%, respectively. All the five cases with anatomical failures showed a complete cicatrization and the ICO movements were poor in all the three cases of functional failure.
Conclusion: The article attempts to standardize the postoperative evaluation of a DCR ostium and provides a systematic protocol and scoring system for possible use by surgeons and researchers alike.
Keywords: DCR, ostium, score, lacrimal, nasal endoscopy
Introduction
External or endoscopic dacryocystorhinostomy (DCR) is the preferred treatment for managing nasolacrimal duct obstructions and dacryocystitis. They are typically associated with high success rates in the order of 80%–95% depending on the underlying etiology.1–8 However, surgical failure occurs and can range from 4% to 13%.1,9–11 Many causes of failure can be attributed to abnormal healing of the ostium, with scarring and cicatricial closure of the osteotomy site being among the most commonly reported.9–12 The other causes of ostium-related failures include inadequate sac exposure, small opening of the sac, cicatrization of the sac prior to surgery, inappropriate location of the ostium, unopened agger nasi cells, removal of sac wall with poor approximation of lacrimal sac and nasal mucosa membranes over the internal common opening (ICO), granulomas, and sump syndrome.10,12,13 The majority of studies published to date assessing post-DCR outcomes have focused on overall ostial measurements or patency testing, with little or no discussion of other ostial-related factors.14–22 This study attempts to establish the influence of various lacrimal ostial factors by outlining a systematic approach to describing and evaluating a postsurgical DCR and presents a novel but yet-to-be-validated scoring system for possible use in clinical and research studies.
Methods
Retrospective evaluation of 125 consecutive lacrimal ostia post-DCR of a single surgeon (MJA) was performed. Institutional review board approval was obtained. Medical records were screened and photographs were assessed to note the details of various ostial parameters, as described subsequently in evaluation of an ostium. Stent removal was performed at 4 weeks. The major time points in evaluation were 4 weeks, 6 weeks, 3 months, and 6 months post-DCR. Anatomical success was defined as a patent ostium on irrigation and functional success as free flow of dye from conjunctival cul-de-sac into the ostium and resolution of epiphora. Surgical success rates were computed, and ostium characteristics in failed cases were studied.
This study has been reviewed by the ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was obtained from the patients.
Evaluation of an ostium
Defining an ostium
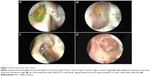
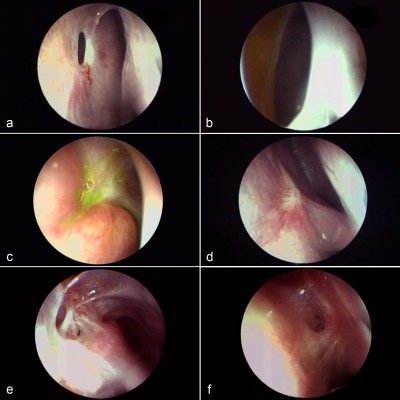
In order to evaluate an ostium, it first must be defined in a consistent manner. We define a DCR ostium as a surgically created opening located in the lateral nasal wall with exposure of the common canaliculus. Its base is mucosal lined, and its edges are described as anterior, posterior, superior, and inferior in relation to the parasagittal plane (Figure 1A and B).
Location of ostium
We describe the location of an ostium in terms of its relation to the middle turbinate (MT), a consistent, prominent, and easy-to-identify landmark in the vicinity. From well-described cadaveric studies,23,24 the most common location of the lacrimal sac is found to be anterior to the axilla of the MT, with two-thirds of the sac length superior to its insertion into the lateral nasal wall. When examining a patient post-DCR, most healed ostia are noted in this location (Figure 1A). However, the observer should be aware that exposing the lacrimal sac may move the original axilla higher up just anterior to the agger nasi cell. This is, however, not an invariable finding, with some ostia occasionally found behind the axilla of MT owing to a forward-protuberant MT, which makes the lacrimal sac appear to be positioned more posteriorly (Figure 1C).
Shape of ostium
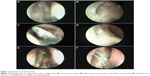
With an ideal healing by primary intention, the ostial edges heal in a radial manner and result in a circular- to oval-shaped ostium with a depressed base (Figure 1B and D). The depression of the base is of particular importance and ideally is shallow, reflecting an appropriately sized osteotomy with bone removal until the sac stands proud of the lateral nasal wall, which after sac opening and good mucosa-to-mucosa apposition heals into a shallow depression (Figure 2A). Deep bases typically result when an inadequately large osteotomy is made, with poor saucerization of their edges. Although they do not typically pose a problem, they can sometimes make it difficult to view the canalicular system on postoperative endoscopy and may be more prone to early crusting and granulomas from excessive bone exposure (Figure 1D). Other less-favorable ostial shapes include crescentric and vertical slits and reflect suboptimal irregular healing and inconsistent patchy cicatrization (Figure 2B). Ideally, when performing a DCR, a surgeon should strive for precision of osteotomy size, mucosal preservation, and flap fashioning in order to achieve circumferential mucosa-to-mucosa apposition, radial healing, and ultimately a shallow-based circular-shaped ostium.
Size of ostium
Previous studies have described numerous techniques of measuring the size of a healed ostium, which include marked Bowman’s probes, olive tip probes, marked suction canulas, scales (Figure 2C), digital subtraction macrodacyrocystography, ultrasonography, and computed tomography scan measurements.14–22
The degree to which an ostium constricts from its initial intra-operative size is variable, with wide reported ranges of 20%–98%.14–22 This variability may not only be influenced by surgical and patient factors but may also reflect different methods employed to create and measure the ostium. Despite the variability described, it is generally thought that with an adequate-sized osteotomy with good mucosa-to-mucosa apposition, the shrink is <25% from the initial ostium created at the time of surgery.19,20
On the basis of published literature,19,20 we propose that at 4 weeks postsurgery, an ostium measuring ≥8×5 mm in size should be considered to be of a good, large size (Figure 1A and D) and an ostium ≤4×3 mm be considered a small one (Figure 2D).
Evolution of an ostium
The evolution of an ostial healing in the postoperative period is an important parameter to monitor (Figure 2E and F). Knowledge of the typical sequence and temporal nature of events in the healing process will allow the surgeon to identify aberrations early and institute corrective measures, where possible. Most of the ostium shrinkage happens in the first 4 weeks and very little, if at all, beyond that.19,20 Regular monitoring helps the surgeon also understand the response to the operative technique and to determine whether there is any need to modify the step(s) of the surgery.
Ostium cicatrix
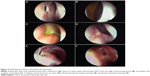
Cicatrization is defined as healing and obliteration of the ostium with a scar tissue. The term “ostium pseudocicatrix” is used when the ostium is covered by a thin layer of scar tissue like a curtain (Figure 3A), while behind this curtain remains a normal ostium. It is important to differentiate this from true cicatrization. In pseudocicatrix, the patient is asymptomatic, and functional endoscopic dye test (FEDT) and irrigation are patent. On endoscopy with a 2.7-mm telescope, there is usually a dehiscence in the obstructing scar curtain (Figure 3A and B) through which the normal ostium or FEDT flow can be appreciated (Figure 3B). Other than pseudocicatrix, irregular healing can lead to incomplete cicatrization (Figure 3C) or a complete cicatricial closure of an ostium (Figure 3D).
Ostial or periostial synechiae
It is important to evaluate any synechiae between the ostium and other nasal structures like turbinates or the septum in the early phases. If they are found to directly threaten the tear flow pathway, synechiolysis may be required. Early detection and management prevents maturation of synechiae. On the basis of the anatomical location and threat, synechiae can be broadly divided into noninterfering (Figure 3E), interfering (Figure 3F), or likely to interfere with ostium functions.
Internal common opening
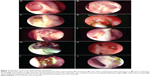
The ICO is the junction between the canaliculi and the lacrimal sac and represents the opening of the distal end of the common canaliculus into the lacrimal sac. The position of the ICO and its dynamicity was evaluated. The most common location in an ideal ostium is on a central or paracentral area of the base (Figure 4A). Occasionally, it is in close relation to one of the four edges (Figure 4B) and uncommonly may be hidden by an overhanging edge (Figure 4C). ICO can be traced by simple visualization of an opening (Figure 4A), by its movements, or by using a dye test (Figure 4C). Less-experienced surgeons can also trace it with the help of a silicone tube. When viewing the ICO, the patient is asked to blink and the dynamic movements of the ICO are studied with the opening and closing of the eyelids. Presence of any obstructive tissues, including membranes or, rarely, granulomas covering the ICO, is noted. These can then be treated with appropriate measures like endocanaliculotomy (Figure 4D).
Stents
Silicone stents are commonly used by many surgeons performing DCRs. Some surgeons use them for all cases, while others rationalize their placement based on canalicular obstructions and sac factors. The presence of stents and the ostium’s response to their presence should be carefully assessed and noted. After debriding any crusts and discharge, commonly associated with stents, the stent is traceable from its distal cut end right up to the ICO (Figure 2F). The dynamicity of the ICO is transmitted to the stents, and it is common to observe the stent moving with each blink of the eyelid. Hence, the stent movements are an indirect indicator of ICO dynamicity. It is important to assess any developing contact granulomas or stent entrapment within healing tissues (Figure 4E).
Functional endoscopic dye test
FEDT is performed by instilling 2% fluorescein drops in the conjunctival cul-de-sac and assessing its natural flow into the ostium with normal blinking. In the presence of a normal-functioning lacrimal pump and patent system, the dye is visualized in the ostium within few seconds (Figure 4C) but usually within a minute (Figures 1A and 3C). The authors do not irrigate unless the patient is symptomatic and FEDT is delayed or negative (no dye in ostium). If no spontaneous flow of dye into the ostium is noted but is witnessed after subsequent irrigation, this indicates lacrimal pump failure or partially obstructed canaliculus. Lack of dye in the ostium on irrigation, coupled with a reflux, indicates a physical obstruction at the ICO or a location proximal to it.
Ostial and periostial granulomas
Ostial granulomas are only occasionally encountered post-DCR, in which a good mucosa-to mucosa approximation is performed. However, aggressive healing or contact granulomas secondary to stents are more common (Figure 1C). Most of the granulomas resolve with topical ocular and nasal steroids. Granulomas threatening the ICO (Figure 4E) or entrapping a stent within them may require a careful surgical removal (Figure 4F).
Other ostium pathologies
There are numerous ostium pathologies or deviations from normal behaviors that need to be identified, monitored, and treated if indicated. Arbitrarily, they can be classified into major and minor. Major pathologies are rare and include soft tissue infection (Figure 4G) of the ostium, orbital breach with fat prolapse toward ostium, and organizing or obstructive tissues threatening the ICO (Figure 4F). Minor pathologies can be diffuse ostium edema (Figure 4H), organizing discharge (Figure 4I), and unwarranted ethmoid entry (Figure 4J).
Results
A total of 125 ostia were evaluated on the aforementioned ostium parameters. Because five ostia showed a complete cicatricial closure with no recognizable features, the remaining 120 ostia were studied. The ostium location was anterior to the axilla of the MT in 85.8% (103/120) of the cases. Furthermore, 76.6% (92/120) of the ostia were circular to oval in shape with a shallow base, followed by 21.6% (26/120) with a circular shape but a deep base. One ostium each showed a crescentric and a vertically slit shape, respectively. The ostium size was good (>8×5 mm) in 78.3% (94/120), small (≤4×3 mm) in 4.1% (5/120), and of intervening sizes in 17.5% (21/120) of the cases. Pseudocicatrix was noted in 3.3% (4/120) and incomplete cicatrix in 0.8% (1/120) of the ostia, and 1.6% (2/120) of the ostia showed noninterfering synechiae. ICO was found to be located in the central or paracentral basal area in 75.8% (91/120), close to the edges in 21.6% (26/120) and with overhanging edges in 2.6% (3/120) of the cases. Granulomas were noted on the edges in 6.6% (8/120) and on the base in 3.3% (4/120) of ostia and were noted to be managed successfully with an excision and silver nitrate base cautery. Ostium complications noted were diffuse edema in 1.6% (2/120) and one case each of soft tissue infections and unwarranted ethmoid entry.
Among the overall 125 cases assessed, anatomical success was achieved in 96% (120/125) and functional success in 93.6% of the cases. All the five cases with anatomical failures showed a complete cicatrization of the ostium with unrecognizable parameters. Two among the anatomical failures had history of complications, one had intraoperative orbital breach with extensive synechiae, and one had postoperative soft tissue infection. Three cases with functional failure showed negative FEDT with patent ostium on irrigation. The ICO movements were poor in all these three cases. Two of the functional failures showed a small ostium and the remaining one showed incomplete cicatrization.
DCR ostium scoring
The DCR ostium scoring system or DOS scoring system is presented using the aforementioned parameters. The purpose of this system is to possibly provide surgeons and researchers alike a yet-to-be validated, easy-to-use system to assess post-DCR ostia. On the basis of the literature14–31 and our evaluation of ostia, ten ostium parameters considered to have a potential influence on the lacrimal system were chosen. This scoring system is an initial attempt and may undergo changes after an ongoing study to validate it is carried out.
The proposed DOS system scores each of the ten ostium parameters with scores ranging from 1 to 4, with 4 reflecting the best-case scenario or the ideal desired parameter, 3 for mild, 2 for moderate, and 1 for severe deviation from the expected normal or 1 being the worst-case scenario. The maximum points that can therefore be achieved for an ostium evaluation are 40, with the minimum being 10. On the basis of the significance of each subparameter, the authors propose that ostia achieving overall DOS scores of 36–40 be graded as excellent, 31–35 as good, 21–30 as fair, and 10–20 as poor (Table 1).
 
|
Table 1 The DCR ostium (DOS) scoring system |
Conclusion
Evaluation of the DCR ostium at regular intervals is important for the surgeon to understand how surgical techniques affect the healing and therefore the success rate of the procedure. Routine ostium evaluation helps the surgeon in the early detection of pathologies and may facilitate early corrective intervention. The DOS scoring system presented here is a prototype of a design aimed to be used in routine clinical evaluation of ostia following a DCR. Further studies focusing on validation of this scoring system are being carried out so that it provides lacrimal surgeons with a standardized objective way for the assessment and comparison of physical and functional outcomes between different approaches and techniques.
Disclosure
Peter John Wormald receives royalties from Medtronic for design of instruments and is a consultant to Neilmed, both not related to this study. The other authors report no conflicts of interest in this work.
References
Tarbet KJ, Custer PL. External dacryocystorhinostomy. Surgical success, patient satisfaction and economic costs. Ophthalmology. 1995;102:1065–1070. | ||
Türkcü FM, Oner V, Taş M, Alakuş F, Işcan Y. Anastomosis of both posterior and anterior flaps or only anterior flaps in external dacryocystorhinostomy. Orbit. 2012;31:383–385. | ||
Cokkeser Y, Evereklioglu C, Er H. Comparative external versus endonasal dacryocystorhinostomy: results in 115 patients. Otolaryngol Head Neck Surg. 2000;123:488–491. | ||
Dolman PJ. Comparison of external dacryocystorhinostomy with non-laser endonasal dacryocystorhinostomy. Ophthalmology. 2003;110:78–84. | ||
Hartikainen J, Grenman R, Puukka P, Seppa H. Prospective randomized comparison of external dacryocystorhinostomy and endonasal laser dacryocystorhinostomy. Ophthalmology. 1998;105:1106–1113. | ||
Tsirbas A, Wormald PJ. Endonasal dacryocystorhinostomy with mucosal flaps. Am J Ophthalmol. 2003;135:76–83. | ||
Davies MJ, Lee S, Lemke S, Ghabrial R. Predictors of anatomical patency following primary endonasal dacryocystorhinostomy: a pilot study. Orbit. 2011;30:49–53. | ||
Codere F, Denton P, Corona J. Endonasal dacryocystorhinostomy: a modified technique with preservation of the nasal and lacrimal mucosa. Ophthal Plast Reconstr Surg. 2010;26:161–164. | ||
Walland MJ, Rose GE. The effect of silicone intubation on failure and infection rates after dacryocystorhinostomy. Ophthalmic Surg. 1994;25:597–600. | ||
McLachlan DL, Shannon GM, Flanagan JC. Results of dacryocystorhinostomy: analysis of the re-operation. Ophthalmic Surg. 1980;11:427–430. | ||
Allen KM, Berlin AJ, Levine HL. Intranasal endoscopic analysis of dacryocystorhinostomy failure. Ophthal Plast Reconstr Surg. 1988;4:143–145. | ||
Welham RA, Wulc AE. Management of unsuccessful lacrimal surgery. Br J Ophthalmol. 1987;71:152–157. | ||
Konuk O, Kurtulmusoglu M, Knatova Z, Unal M. Unsuccessful lacrimal surgery: causative factors and results of surgical management in a tertiary referral center. Ophthalmologica. 2010;224:361–366. | ||
Linberg JV, Anderson RL, Bumsted RM, Barreras R. Study of intranasal ostium at external dacryocystorhinostomy. Arch Ophthalmol. 1982;100:1758–1762. | ||
Ezra EJ, Restori M, Mannor GE, Rose GE. Ultrasonic assessment of rhinostomy size following external dacryocystorhinostomy. Br J Ophthalmol. 1998;82:786–789. | ||
Ben Simon GJ, Brown C, McNab AA. Larger osteotomies results in larger ostia in external dacryocystorhinostomy. Arch Facial Plast Surg. 2012;14:127–131. | ||
Argin A, Görür K, Ozcan C, Arslan E, Ozmen C, Vayisoglu Y. The role of larger osteotomy in long term success in external dacryocystorhinostomy. J Plast Reconstr Aesthet Surg. 2008;61:615–619. | ||
Baldeschi L, Nolst Trenité GJ, Hintschich C, Koornneef L. The intranasal ostium after external dacryocystorhinostomy and the internal opening of lacrimal canaliculi. Orbit. 2000;19:81–86. | ||
Mann BS, Wormald PJ. Endoscopic assessment of the DCR ostium after endoscopic surgery. Laryngoscope. 2006;116:1172–1174. | ||
Chan W, Selva D. Ostium shrinkage after endoscopic dacryocystorhinostomy. Ophthalmology. 2013;120:1693–1696. | ||
Rootman D, DeAngelis D, Tucker N, Wu A, Hurwitz J. Cadaveric anatomical comparison of the lateral nasal wall after external and endonasal DCR. Ophthal Plast Reconstr Surg. 2012;28:149–153. | ||
Yazici B, Yacizi Z. Final nasolacrimal ostium after external dacryocystorhinostomy. Arch Ophthalmol. 2003;121:76–80. | ||
Wormald PJ, Kew J, Van Hasselt CA. The intranasal anatomy of the nasolacrimal sac in endoscopic dacryocystorhinostomy. Otolaryngol Head Neck Surg. 2000;123:307–310. | ||
Rebeiz E, Shapshay S, Bowlds J, Pankratov MM. Anatomical guidelines for dacryocystorhinostomy. Laryngoscope. 1992;102(10):1181–1184. | ||
Kamal S, Ali MJ, Naik MN. Circumostial injection of MMC (COS MMC) in external and endoscopic dacryocystorhinostomy: efficacy, safety profiles and outcomes. Ophthal Plast Reconstr Surg. 2014;30:187–190. | ||
Lee A, Ali MJ, Li EY, Wong AC, Yuen HK. Balloon dacryoplasty in internal ostium stenosis following endoscopic dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2014;30:7–10. | ||
Ali MJ, Joshi DS, Naik MN, Honavar SG. Clinical profile and management outcomes of acute dacryocystitis: two decades of experience in a tertiary eye care center. Semin Ophthalmol. 2013. Epub 2013 Oct 30. | ||
Tsirbas A, Wormald PJ. Agger nasi cell mucosal autograft for lacrimal sac reconstruction during endonasal dacryocystorhinostomy. Orbit. 2004;23:105–110. | ||
Roithmann R, Burman T, Wormald PJ. Endoscopic dacryocystorhinostomy. Braz J Otorhinolaryngol. 2012;78:113–121. | ||
Tsirbas A, Davis G, Wormald PJ. Mechanical endonasal dacryocystorhinostomy versus external dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2004;20:5–56. | ||
Tsirbas A, Wormald PJ. Mechanical endonasal dacryocystorhinostomy with flaps. Br J Ophthalmol. 2003;87:43–47. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.