Back to Journals » Vascular Health and Risk Management » Volume 10
Congenital anomaly of the inferior vena cava and factor V Leiden mutation predisposing to deep vein thrombosis
Authors Lamparello B, Erickson C, Kulthia A, Virparia V, Thet Z
Received 16 April 2014
Accepted for publication 6 June 2014
Published 4 November 2014 Volume 2014:10 Pages 609—613
DOI https://doi.org/10.2147/VHRM.S66283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Video abstract presented by Brooke M Lamparello.
Views: 1553
Brooke M Lamparello,1,* Cameron R Erickson,2,* Arun Kulthia,3 Vasudev Virparia,3 Zeyar Thet3
1St George’s University, Grenada, West Indies; 2Northeast Ohio Medical University, Rootstown, OH, USA; 3Department of Medicine, Coney Island Hospital, Brooklyn, NY, USA
*These authors contributed equally to this work
Abstract: A previously healthy 21-year-old man presented with back pain, bilateral extremity pain, and right lower extremity weakness, paresthesias, and swelling. Sonographic examination revealed diffuse deep vein thrombosis (DVT) in the femoral and popliteal venous system. CT imaging revealed hypoplasia of the hepatic inferior vena cava (IVC) segment with formation of multiple varices and collateral veins around the kidneys. Hematologic workup also discovered a factor V Leiden mutation, further predisposing the patient to DVT. The rare, often overlooked occurrence of attenuated IVC, especially in the setting of hypercoagulable state, can predispose patients to significant thrombosis.
Keywords: inferior vena cava (IVC), deep vein thrombosis (DVT), lower extremities, thrombophilic, venography
Introduction
Congenital inferior vena cava (IVC) anomalies are an unusual and often underreported and asymptomatic finding that may predispose patients to deep vein thrombosis (DVT). The most common causes of IVC obstruction are malignant neoplasms and IVC thrombosis.1–3 Formation of the IVC occurs during weeks 6 through 8 of embryogenesis. The IVC is formed by continuous formation and regression of three paired embryonic veins: the posterior cardinal, subcardinal, and the supracardinal veins. IVC anomalies occur when there is an abnormal regression or persistence of any of these embryonic veins.4 IVC anomalies have an estimated prevalence of 0.5%–0.6% in healthy individuals and 2% in those with congenital cardiac defects.5
Discovery of an anomalous IVC is typically an incidental finding during medical imaging, cardiac catheterization, or aortoiliac surgery.4,6–11 While imaging is a reliable method to investigate IVC anomalies, most radiologists and clinicians may lack sufficient knowledge of these anomalies. This may lead to misdiagnosis in some circumstances.12
Results
A previously healthy 21-year-old male presented with a 1-week history of back pain and 1-day history of bilateral pain and lower extremity swelling. He also complained of accompanying feelings of numbness, tingling, and tightness in his right leg. His back pain acutely developed 1 week after vigorous weight-training squat exercises. His medical history is significant for an episode of pancreatitis. He was previously a social smoker and reported smoking 1–2 cigarettes on weekends for 3–4 years, but quit 4 months prior to presentation. His family medical history is significant for varicose veins in his grandmother.
On initial physical examination, his right leg was markedly more swollen and edematous than his left. A right lower extremity venous Doppler ultrasound documented diffuse acute thrombosis in the femoral and popliteal venous system. Acute thrombi were visualized in his greater saphenous, superficial femoral, popliteal, and posterior tibial veins. Doppler ultrasound of the left leg revealed normal color and spectral flow in the common femoral, greater saphenous, superficial femoral, popliteal, and posterior tibial veins without evidence of a filling defect or compressibility. A spiral chest CT scan with contrast was performed, which ruled out an acute pulmonary embolism (Figure 1). The CT scan documented attenuation of the hepatic IVC segment and multiple varices and collateral veins in the retroperitoneum around the kidneys, the expected location of the IVC, and collateral vessels in the left anterior chest wall (Figures 2 and 3).
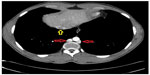  | Figure 1 Chest CT with contrast. Azygos-hemiazygos (red arrows) and the hepatic portion of the IVC (yellow arrow). |
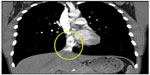  | Figure 2 Hepatic IVC (circled). |
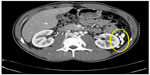  | Figure 3 CT scan with contrast. |
Consequently, a right lower extremity ascending venogram was performed via the right popliteal vein with the patient in prone position (Figure 4). The venogram confirmed Doppler imaging and the diagnosis of extensive venous thrombosis involving the right superficial femoral, common femoral, and right iliac veins. A thrombus was also noted involving the left iliac vein.
  | Figure 4 Lower extremity venogram. Interrupted IVC (yellow arrow) and lower extremity drained by hemiazygos veins (red arrow). |
Laboratory findings were noncontributory, except for a heterozygous factor V Leiden mutation found on thrombophilia workup.
The patient was treated with a catheter-assisted mechanical thrombectomy. The patient also received a power-pulse spray using 8 mg tissue plasminogen activator (TPA) and an additional 2 mg TPA via the infusion catheter. Balloon maceration of the thrombus was performed using a 10 mm ×4 cm balloon catheter. The patient was effectively administered catheter-directed thrombolysis with TPA at 1 mg/hour over a 24-hour period. The patient was also administered heparin at a rate of 300 U/hour.
Postlytic venography confirmed successful thrombolysis of the right lower extremity veins and the presence of an anomalous IVC. Venography revealed the lower extremities were properly being drained by the azygos/hemiazygos venous system. The patient was anticoagulated and discharged home with recommendation for long-term warfarin therapy. The patient was also advised not to engage in vigorous lower extremity exercises.
Discussion
In the case of our patient, with an interrupted hepatic IVC, the anomaly results from the embryologic failure of the anastomosis of the subcardinal vein–hepatic segment. The hepatic veins converge to flow directly into the right atrium, and the lower extremity blood returns to the superior vena cava through the azygos/hemiazygos venous system.13
When treating patients with congenital IVC defects, it is important to recognize the anomaly as a risk factor for DVT. The estimated prevalence of DVT in patients under 30 years of age with absent IVC is 5% versus 0.5%–0.6% in the general healthy population.14,15 Ultrasound, the standard diagnostic test for DVT, does not accurately visualize the abdominal veins, since they cannot be accurately compressed.14–16 When younger patients present with idiopathic DVT, in the absence of clotting defects or factors known to predispose individuals to thrombophilia, clinicians must consider an underlying IVC anomaly as the cause for DVT development.5,14 Our patient is an interesting case because he had both a clotting disorder and an IVC anomaly, which provided a challenge when discerning the exact etiology of his symptoms. The presence of both factors likely contributed to our patient’s thrombotic event. However, there has yet to be substantial research to uncover the precise nature of such a relationship. The presence of both an IVC anomaly and clotting disorder warranted the patient’s requirement of long-term anticoagulation following thrombolysis to prevent recurrent DVT and sequelae. Elastic stockings have frequently been reported as adjuvant therapy to prevent stasis ulcers and venous insufficiency.17 Our patient’s symptoms appeared only after strenuous activity and he had no previous history of symptoms indicative of DVT or venous insufficiency. Therefore, we did not feel elastic stockings needed to be utilized at this time given the resolution of his symptoms following thrombolysis. The patient was advised to maintain close follow-up and that elastic stockings may be used in the future should they be warranted.
Our patient also presented with neurological symptoms that could not solely be attributed to the presence of DVT. Epidural vein enlargement due to IVC anomalies is a rare but well-documented pathological process that can result in nerve root compression syndrome.18–21 It is likely that our patient also experienced some degree of nerve compression or irritation due to epidural vein expansion. However, magnetic resonance imaging (MRI) – the imaging modality necessary for evaluation – was not performed. When patients present with symptoms of nerve root compression, sciatica, exercise-induced numbness, or lower back pain with no other pathologic explanation, full radiologic workup is used to assess epidural varicosis secondary to IVC anomalies – particularly if patients also present with symptoms of DVT.
Ruggeri et al14 suggest the development of DVTs may be due to the inadequate drainage of the lower limbs by the compensating azygos venous system despite enlargement of the vessels. IVC anomalies also predispose patients to DVT at an earlier age. Obernosterer et al22 found patients with anomalies of the IVC presenting with DVT were significantly younger (25±6 years) than those without (53±19 years; P=0.002). As in the case of our patient, strenuous activity has been documented as a precipitating factor in DVT formation in young, otherwise healthy individuals with an IVC anomaly.6,22–24 Therefore, patients with a diagnosed IVC anomaly should be advised against excessive exercise or demanding physical activity. We hypothesize that the relationship between arduous physical activity and DVT in patients with IVC anomalies may not be established as a direct causal one. However, frequent stretching of the IVC could possibly result in endothelial damage and postinflammatory stenosis of the IVC, potentially rendering it susceptible to the development of thrombosis. It has also been suggested that collaterals developed in patients with IVC anomalies provide insufficient venous drainage during times of increased blood flow, such as arduous physical activity.17 We suggest the combination of postinflammatory stenosis due to recurring IVC stretching and stasis due to inadequate collateral drainage aided in creating a milieu conducive to thrombus formation. However, more research must be conducted to determine the exact pathophysiological mechanism of action.
In those patients found to have an IVC anomaly, clinicians must also evaluate patients for renal hypoplasia or horseshoe kidney. Gayer et al25 identified nine patients with complete absence of the IVC and a hypoplastic right kidney. While right renal hypoplasia is more commonly associated with IVC anomalies, there has been one previous report of left renal hypoplasia and aberrant IVC.26 Van Veen et al26 has proposed the clinical constellation of kidney and IVC abnormalities with associated DVT to be named kidney IVC abnormalities leg thromboses (KILT) syndrome.
Conclusion
Since an anomalous IVC cannot be detected by ultrasonography, the ideal imaging modality is using CT with contrast, MRI, or venography. Clinicians should suspect and rule out IVC anomalies if a patient presents with lower limb venous stasis, chronic venous hypertension in the lower extremities, and thrombosis. The diagnosis can also be supported if the intrathoracic hemiazygos or azygos systems are dilated.16
Acknowledgments
This material is based upon work supported with the resources and the use of facilities at Coney Island Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
Simpson AH, Kilby JO. Inferior vena cava thrombosis following a cycle ride. J Roy Soc Med. 1988;81(12):738–739. | |
Dale WA, Allen TR. Unusual problems of venous thrombosis. Surgery. 1975;78(6):707–722. | |
Stiris MG, Finnanger AM. Postrenal segment aneurysm and intrahepatic interruption of the inferior vena cava. A case report. Acta Radiol. 1987;28(5):545–548. | |
Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20(3):639–652. | |
Anderson RC, Adams P Jr, Burke B. Anomalous inferior vena cava with azygos continuation (infrahepatic interruption of the inferior vena cava). Report of 15 new cases. J Pediatr. 1961;59:370–383. | |
Gayer G, Luboshitz J, Hertz M, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol. 2003;180(3):729–732. | |
Minniti S, Visentini S, Procacci C. Congenital anomalies of the venae cavae: embryological origin, imaging features and report of three new variants. Eur Radiol. 2002;12(8):2040–2055. | |
Ueda J, Hara K, Kobayashi Y, Ohue S, Uchida H. Anomaly of the inferior vena cava observed by CT. Comput Radiol. 1983;7(3):145–154. | |
Bastonuis E, Pikoulis E, Leppäniemi A, Maltezos C, Milas F, Alexiou D. Anomalous inferior vena cava complicating abdominal aortic aneurysmectomy. J Cardiovasc Surg (Torino). 1997;38(4):367–369. | |
Weiner RI, Maranhao V, Levin HB, Aji J, Blaker A, Levine HB. Anomalous inferior vena cava with azygos continuation. J Am Osteopath Assoc. 1993;93(7):775–777. | |
Guerra Ramos JM, Font ER, Moya i Mitjans A. Radiofrequency catheter ablation of an accessory pathway through an anomalous inferior vena cava with azygos continuation. Europace. 2004;6(2):134–137. | |
Evans JC, Earis J, Curtis J. Thrombosed double inferior vena cava mimicking paraaortic lymphadenopathy. Br J Radiol. 2001;74(878):192–194. | |
Qian Z, Yang MF, Zuo KQ, Cheng J, Xiao HB, Ding WX. Computed tomography manifestations of common inferior vena cava dysplasia and its clinical significance. Exp Ther Med. 2013;5(2):631–635. | |
Ruggeri M, Tosetto A, Castaman G, Rodeghiero F. Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet. 2001;357(9254):441. | |
Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol. 2001;114(4):878–880. | |
Iqbal J, Nagaraju E. Congenital absence of inferior vena cava and thrombosis: a case report. J Med Case Rep. 2008;2:46. | |
Lambert M, Marboeuf P, Midulla M, et al. Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literature. Vasc Med. 2010;15(6):451–459. | |
Endres S. Epidural varicosis as a possible cause of radicular pain: a case report. J Med Case Rep. 2011;5:537. | |
Dudeck O, Zeile M, Poellinger A, Kluhs L, Ludwig WD, Hamm B. Epidural venous enlargements presenting with intractable lower back pain and sciatica in a patient with absence of intrarenal inferior vena cava and bilateral deep venous thrombosis. Spine (Phila Pa 1976). 2007;32(23):E688–E691. | |
Yugueros X, Alvarez B, Fernández E, Boqué M, Matas M. Compressive symptoms due to thrombosed or hypertrophic collateral circulation in infrarenal inferior vena cava agenesis. Ann Vasc Surg. 2013;27(2):238. e9–238. e13. | |
Kamerath J, Morgan WE. Absent inferior vena cava resulting in exercise-induced epidural venous plexus congestion and lower extremity numbness: a case report and review of the literature. Spine (Phila Pa 1976). 2010;35(18):E921–E924. | |
Obernosterer A, Aschauer M, Schnedl W, Lipp RW. Anomalies of the inferior vena cava in patients with iliac venous thrombosis. Ann Intern Med. 2002;136(1):37–41. | |
Arao M, Ogura H, Ino T, et al. Unusual inferior vena cava obstruction causing extensive thrombosis: an intravascular endoscopic observation. Intern Med. 1993;32(11):861–864. | |
Dean SM, Tytle TL. Acute right lower extremity iliofemoral deep venous thrombosis secondary to an anomalous inferior vena cava: a report of two cases. Vasc Med. 2006;11(3):165–169. | |
Gayer G, Zissin R, Strauss S, Hertz M. IVC anomalies and right renal aplasia detected on CT: a possible link? Abdom Imaging. 2003;28(3):395–399. | |
Van Veen J, Hampton KK, Makris M. Kilt syndrome? Br J Haematol. 2002;118(4):1199–1200. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
