Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Combined application of eicosapentaenoic acid and docosahexaenoic acid on depression in women: a meta-analysis of double-blind randomized controlled trials
Authors Yang J, Han D, Qiao Z, Tian X, Qi D, Qiu X, Han Y, Zhou X
Received 14 April 2015
Accepted for publication 18 June 2015
Published 10 August 2015 Volume 2015:11 Pages 2055—2061
DOI https://doi.org/10.2147/NDT.S86581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Wai Kwong Tang
Jia-run Yang, Dong Han, Zheng-xue Qiao, Xue Tian, Dong Qi, Xiao-hui Qiu
Department of Medical Psychology, Public Health Institute of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China
Objectives: Previous randomized controlled trials (RCTs) suggest that depression can be effectively treated by omega-3 polyunsaturated fatty acids (PUFAs). Therefore, we conducted this meta-analysis to systematically evaluate the clinical applicability of the combination of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are the two major bioactive types of PUFAs, in depressed women.
Methods: RCTs that compared the combination of DHA and EPA to placebo for short-course treatment of depression in women were systematically reviewed up to March 2015. Outcome measurement was the standardized difference in means in clinical measure of depression severity. Random effect model was performed. Meta-regression analysis was performed to assess the effects of baseline depression scores.
Results: Data were obtained from eight RCTs. In these RCTs, 182 patients received placebo and 185 patients received DHA and EPA. The pooled standardized difference in mean was 0.65 with 95% CI = [0.18, 1.12]. There was no relation between the efficacy and the baseline depression scores. The sensitivity analysis found that the combination of EPA and DHA as monotherapy yielded a standardized difference in means of 0.65 (95% CI =0.41, 0.90) without heterogeneity.
Discussion: These results indicate a beneficial effect of the combination of EPA and DHA on depressed mood in women compared with placebo. The clinical applicability of EPA and DHA showed greater promise and should be further explored.
Keywords: depression, omega-3 polyunsaturated fatty acids, PUFAs, docosahexaenoic acid, DHA, eicosapentaenoic acid, EPA
Introduction
Depression is one of the most debilitating mental disorders in psychiatry field, which affects more than 10% of the population and results in more than 10% of the global disease burden.1–3 There are about 10%–20% free-living elderly subjects and 20%–30% elderly hospitalized patients experiencing the depressive symptoms.4 Currently, despite a large number of established medication options for depression, about 19%–34% of patients fail to respond to the first-line treatments.5 On the other hand, the female preponderance of depression and its emergence in adolescence was reported by most of the studies.6 Studies reported that the prevalence of depression was doubled in girls than boys between 15 years and 19 years,7 and this trend persisted until 54 years.8 Some studies reported a higher prevalence of depression in women than men, even after menopause.9 This sex disparity indicated that gonadal hormones might play a potential role in the etiology of depression. Additionally, some data have raised the concern that using selective serotonin reuptake inhibitor antidepressants during the pregnancy may result in self-limited neonatal behavioral syndrome.10 Based on these results, there is an urgent need to identify a new treatment for depression in women.
Omega-3 polyunsaturated fatty acids (PUFAs), including the two major bioactive types: docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are nutritional compounds, which are not synthesized by the human body.11,12 Omega-3 PUFAs are considered a necessary dietary component. Some studies reported that the deficit of DHA, EPA, or both in cell membranes or plasma was associated with an increased incidence of depression.13,14 The decreased levels of DHA and/or EPA were found in depressed patients.15 Furthermore, researchers found that PUFAs may be beneficial in individuals with diagnosed depressive illness.16 However, previous studies have demonstrated inconsistent findings regarding the efficacy of DHA and/or EPA in treating depression in women.17,18 Freeman et al reported that there was no significant difference between omega-3 PUFAs and placebo in treating depression in women.17 But Rondanelli et al reported that the supplementation of omega-3 PUFAs had therapeutic benefits in women with depression.18 These discrepant conclusions may be due to a lack of statistical power among some of the individual clinical trials.19 Therefore, this work seeks to examine the efficacy of DHA and EPA on treating depression in women. We hypothesized that the combined application of DHA and EPA could yield antidepressant effect on women with depression. This meta-analysis should obtain a more accurate conclusion by integrating the results from multiple studies.20
Methods
Study selection
The first step of this meta-analysis was to identify eligible trials. Electronic searches were performed in international databases (PubMed, Cochrane Controlled Trials Register, Web of Science, Embase), two Chinese databases (China Biology Medicine disc, China National Knowledge Infrastructure), and relevant websites dated up to December 2014. The search terms used were “depress*” combined with “omega-3”, “PUFA”, “polyunsaturated fatty acids”, “DHA”, and “EPA”. In order to mitigate language bias, no language restriction was imposed. Reference documents listed in relevant papers, conference summaries, and the International Clinical Trials Registry Platform were also researched.
We selected studies for subsequent analysis according to the following inclusion criteria: 1) randomized controlled trials (RCTs) comparing omega-3 fatty acid and placebo (active or not); 2) women patients over 18 years of age; 3) informed consent provided; 4) outcome assessed by Montgomery–Åsberg Depression Rating Scale, Hamilton Depression Rating Scale (HDRS), Clinical Global Impression, Beck Depression Inventory, or Geriatric Depression Scale; and 5) used EPA and DHA at the same time. Meanwhile, studies according with any of the following criteria were excluded: 1) nonrandom allocation; 2) case reports and reviews; and 3) duplicate studies.
Data extraction
Two reviewers independently verified all potentially suitable studies by the inclusion/exclusion criteria and completed the data abstraction. Any disagreement was solved by consensus, and if needed, a third reviewer was consulted. Data extracted from the included studies were recorded in a structured fashion as follows: 1) patients characteristics (ie, mean age, mean depression score, country, and outcome measurement); 2) treatment parameters (ie, treatment time, treatment strategy, and daily dose); and 3) outcome measures (ie, mean score of depression after the intervention and dropouts). When the trials reported results from different kinds of rating scales, HDRS was preferentially selected. For data that could not be directly extracted from the study, the data were retrieved through correspondence with the primary author or from other studies.
Bias risk assessment
Two reviewers independently assessed the quality of each included RCTs according to the Cochrane Collaboration criteria.21 Bias risk was assessed by the following items: the quality of randomization, using the allocation concealment, blinding the investigators and patients, and reporting incomplete data and similar baseline clinical characteristics. Studies with two or more bias risks were excluded from the subsequent meta-analysis.
Statistical analysis
RevMan 5.1 (Cochrane Information Management System [IMS]) and Stata software 8.0 (StataCorp LP, College Station, TX, USA) were used to do the statistical analyses. Continuous data were extracted as mean and standard deviations (SDs). All depression scales’ means and SDs at the beginning of treatment and after treatment in two groups were combined.22 To correct for small sample bias, the standardized mean effect for included RCTs was calculated by using Hedges’ g.23 We used the Mantel–Haenszel random-effects model, as it was assumed that the included RCTs probably had varying true treatment effects.24 But if there was no heterogeneity, the fixed-effects model was used. If needed, subgroup analysis and sensitivity analysis were conducted. Heterogeneity was investigated using the Q-statistic and I2.25 The Egger test and funnel plots were conducted to assess the potential presence of publication bias. The protocol of this work followed the recommendations for conducting a meta-analysis.26
Results
Totally, 254 potentially relevant studies were obtained in the initial Internet search. Among these, we first excluded 76 studies by reviewing the titles. Second, we excluded 167 additional studies by reviewing the abstract. Third, we excluded eleven additional studies by two reviewers independently reviewing the full texts. Finally, eight RCTs met all the inclusion/exclusion criteria and were used for the subsequent analysis (Figure 1). Although the references listed behind these studies were researched for possibly omitted RCTs, no more RCTs were found.
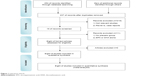  | Figure 1 Literature search. |
Characteristics of the eight studies
These eight RCTs contained an aggregate of 367 adult patients, composed of 182 patients receiving placebo and 185 patients receiving EPA and DHA.17,18,27–32 Three RCTs used paraffin oil as placebo;17,18,31 two RCTs used olive oil ethyl esters as placebo;27,32 and another three RCTs used sunflower oil, corn oil, and Sunola oil as placebo.24,29,30 The dose of EPA was higher than that of DHA in seven RCTs, but the dose of DHA (1.64 g) was higher than that of EPA (0.42 g) in Rees et al.29 Six RCTs used EPA and DHA as monotherapy,18,27–31 and two RCTs used them as an augmentation agent (supportive psychotherapy,17 tert-butylhydroquinone and tocopherols32). Patients in seven RCTs received 8 weeks of treatment, but patients in Rees et al received 6 weeks of treatment.29 The age of all patients in three RCTs was more than 65 years.18,28,31 The detailed information is described in Tables 1 and 2.
  | Table 2 Treatment parameters of included randomized controlled trials |
Bias risk assessment
In this review, the eight studies used randomization and conducted allocation concealment. All studies reported the similar baseline characteristics and incomplete data. As far as we know, in clinical studies, the double-blind was difficult, but all these included RCTs blinded the investigators and patients. Thus, the eight RCTs in this review were consistently of high quality. As these RCTs displayed no bias risk, all RCTs were used in this meta-analysis (Table 3).
  | Table 3 Bias risk of included studies |
Standardized difference in means
All the included RCTs reported the data at the treatment end point.17,18,27–32 The pooled standardized difference in means was 0.52 (95% CI =0.31, 0.73) for the fixed-effects model and 0.65 (95% CI =0.18, 1.12) for the random-effects model (Figure 2), which indicated a beneficial effect of the combination of EPA and DHA on depressed mood compared with placebo. But, significant heterogeneity in effect size existed (P<0.0001, I2=78%). In order to check whether or not the depression scores on baseline played a role in the efficacy of the combination of EPA and DHA, we performed a meta-regression analysis. The results indicated that there was no relation between the efficacy and the baseline depression scores (regression coefficient =0.015; 95% CI =-0.007, 0.034; P=0.24). No significant asymmetry in the inverted funnel plots of these RCTs appeared. Meantime, the Egger test was conducted, and the result showed that the outcome was not influenced by the publication bias (P=0.32).
Sensitivity analysis
Two RCTs used the combination of EPA and DHA as an augmentation agent.17,32 Then, we excluded these two RCTs to investigate the efficacy of EPA and DHA as monotherapy. The results showed that the pooled standardized difference in means was 0.65 (95% CI =0.41, 0.90) for the fixed-effects model (Figure 3). This exclusion did not significantly affect the initial effect size estimates for the whole sample, but no significant heterogeneity existed (P=0.33, I2=13%). One study recruited four (18%) men with major depression disorder (MDD), and one study treated the recruited patients for six weeks. Then, we excluded these two RCTs to do sensitivity analysis, respectively. The results showed that the exclusion did not significantly affect the initial effect size estimates.
Subgroup analysis
Three RCTs mainly used Geriatric Depression Scale to assess the outcome,18,28,31 and five RCTs mainly used HDRS to assess the outcome.17,27,29,30,32 Then we did subgroup analysis according to the outcome measurement. The results showed that the five RCTs and three RCTs yielded the standardized difference in means of 0.34 (95% CI =0.07, 0.61) and 0.82 (95% CI =0.47, 1.17), respectively. Three RCTs recruited patients with an average age of above 80 years18,28,31 and five RCTs recruited patients with an average age of 30–50 years.17,27,29,30,32 Then we did subgroup analysis according to the age. The results showed that the three RCTs and five RCTs yielded the standardized difference in means of 0.34 (95% CI =0.07, 0.61) and 0.82 (95% CI =0.47, 1.17), respectively. These results indicated that the different outcome measurement methods and age did not influence the outcomes.
Discussion
This meta-analysis first investigated the efficacy of EPA and DHA in the treatment of depressed women. Compared to the placebo, the combination of EPA and DHA had significantly better efficacy with pooled standardized difference in means of 0.65 (95% CI =0.18–1.12, z=2.70, P=0.007), but significant heterogeneity existed (P<0.0001, I2=78%). After excluding the two studies that used the combination of EPA and DHA as an augmentation agent, the pooled standardized difference in means had no changed, but significant heterogeneity non-existed (P=0.33, I2=13%). These results showed that the combination of EPA and DHA could effectively treat the depression in women.
Mischoulon et al hypothesized that subjects with diets low in PUFAs would have higher baseline severity of depression.33 The association between depression and PUFAs has been reported by several studies.34,35 One study reported that the DHA levels were particularly lower in the postmortem orbitofrontal cortex of female patients with major depressive disorder than their male counterparts.36 Another study using adipose tissue, which reflects long-term dietary fat intake, also found a significantly lower DHA concentrations in female MDD patients than male MDD patients.37 The reason might be the conversion of alpha-linolenic acid to DHA, positively regulated by estrogen.38 Meanwhile, many evidences have implicated estrogen in the pathophysiology of MDD in women.39,40 Furthermore, another study found that the low levels of DHA could predict the low levels of 5-hydroxytryptamine, which was implicated in the pathophysiology of depression.41 This work found that the supplementation of PUFAs could yield better efficacy for women with depression. Moreover, all the included studies showed that PUFAs were well tolerated by postpartum and pregnant women.
Seven of eight studies in this meta-analysis used the larger dose of EPA than DHA. Previous study showed that EPA had an effect on core depressive symptoms, such as guilt feelings, depressed mood, and worthlessness as well as insomnia.42 Mischoulon et al reported that the observed decrease in HAM-D-17 score was significant for the EPA group, but not for the placebo group.33 They also found that the administration of EPA resulted in a significant increase in mean plasma EPA, but had limited effect on plasma DHA. The mechanism of EPA and DHA was different. The EPA might influence mood by acting through eicosanoid mechanisms to increase cerebral blood flow. The brains contained much more DHA than EPA, which might partly explain why many studies used the larger dose of EPA than DHA.
Depression comorbidity with other diseases, such as cardiovascular diseases and dementia, in the elderly population often lower the effects of antidepressive treatments. This might be caused by the age or the comorbidity. In this meta-analysis, we found that the combined application of EPA and DHA in younger women with depression yielded a better antidepressant effect than that in elderly women with depression (standardized difference in means, 0.82 versus 0.34). Although these results were limited by the relatively small studies, the treatments for elderly women with depression needed more attention.
Several limitations should be addressed here: 1) the number of included RCTs and depressed patients were relatively small; 2) the dose of EPA and DHA, the age of included patients, and the used placebo were not exactly the same and there were also the general problems for metastudies to solve; 3) four of 367 included patients were men; and 4) whether or not it is appropriate to apply this conclusion to postpartum depression and male with depression or vascular depression is unknown. However, this pooled analysis of eight double-blinded RCTs found that the clinical applicability of EPA and DHA showed greater promise and should be further explored.
Acknowledgments
The study was supported by the grant from the Scientific Research Foundation of the Educational Department of Heilongjiang Province (1231225) and the China Postdoctoral Science Foundation (2014M551274) to Xiao-hui Qiu and the National Natural Science Foundation of China (81302484) to Zheng-xue Qiao.
Disclosure
The authors declare no conflicts of interest in this work.
References
Safa M, Fallah Tafti S, Ghassem Boroujerdi F, Talischi F. Clinical trial in the treatment of 80 Iranian patients with major depression disorder by the combination of omega 3 fatty acid and a selective serotonin reuptake inhibitor. Ther Adv Psychopharmacol. 2013;3(4):186–190. | ||
Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–3105. | ||
Reynolds E. Brain and mind: a challenge for WHO. Lancet. 2003;361:19–24. | ||
Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–311. | ||
Fava M. Diagnosis and de finition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. | ||
Essau CA, Lewinsohn PM, Seeley JR, Sasagawa S. Gender differences in the developmental course of depression. J Affect Disord. 2010;127(1–3):185–190. | ||
Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57(1):21–27. | ||
Cairney J, Wade TJ. The influence of age on gender differences in depression: further population-based evidence on the relationship between menopause and the sex difference in depression. Soc Psychiatry Psychiatr Epidemiol. 2002;37(9):401–408. | ||
Bijl RV, De Graaf R, Ravelli A, Smit F, Vollebergh WA. Gender and age-specific first incidence of DSM-III-R psychiatric disorders in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Soc Psychiatry Psychiatr Epidemiol. 2002;37(8):372–379. | ||
Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. | ||
Torpy JM, Lynm C, Glass RM. JAMA patient page. Eating fish: health benefits and risks. JAMA. 2006;296:1926. | ||
Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. | ||
Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis: possible candidate genes. Prostaglandins Leukot Essent Fatty Acids. 1999;60:217–234. | ||
Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213. | ||
Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55(9):891–896. | ||
Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–770. | ||
Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110:142–148. | ||
Rondanelli M, Giacosa A, Opizzi A, et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2010;29:55–64. | ||
Maxwell SE, Kelley K, Rausch JR. Sample size planning for statistical power and accuracy in parameter estimation. Annu Rev Psychol. 2008;59:537–563. | ||
Huf W, Kalcher K, Pail G, Friedrich ME, Filzmoser P, Kasper S. Meta-analysis: fact or fiction ? How to interpret meta-analyses. World J Biol Psychiatry. 2011;12:188–200. | ||
Higgins JPT, Altman DG. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2011:187–243. | ||
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. | ||
deeks JJ, Altman DG, Bradbrun MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. London: BMJ Publishing Group; 2001:285–312. | ||
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Br Med J. 2011;342:d549. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. | ||
Sacks HS, Berrier J, Reitman D, Ancona-Berk V, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316:450. | ||
Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644–651. | ||
Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, et al. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. J Nutr Health Aging. 2011;15:37–44. | ||
Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42:199–205. | ||
Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr. 2009;89:641–651. | ||
Rizzo AM, Corsetto PA, Montorfano G, et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J. 2012;11:82. | ||
Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(4):267–271. | ||
Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009;70(12):1636–1644. | ||
Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393–1396. | ||
Hibbeln JR, Salem N Jr. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. | ||
McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62(1):17–24. | ||
Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. | ||
Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. | ||
Grigoriadis S, Kennedy SH. Role of estrogen in the treatment of depression. Am J Ther. 2002;9:503–509. | ||
Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–174. | ||
Hibbeln JR, Umhau JC, Linnoila M, et al. A replication study of violent and nonviolent subjects: cerebrospinal fluid metabolites of serotonin and dopamine are predicted by plasma essential fatty acids. Biol Psychiatry. 1998;44(4):243–249. | ||
Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159(3):477–479. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



