Back to Journals » OncoTargets and Therapy » Volume 7
Combination therapy with brentuximab vedotin and cisplatin/cytarabine in a patient with primarily refractory anaplastic lymphoma kinase positive anaplastic large cell lymphoma
Authors Heidegger S, Beer A, Geissinger E, Rosenwald A, Peschel C, Ringshausen I, Keller U
Received 3 January 2014
Accepted for publication 21 February 2014
Published 20 June 2014 Volume 2014:7 Pages 1123—1127
DOI https://doi.org/10.2147/OTT.S59795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Simon Heidegger,1 Ambros Beer,2 Eva Geissinger,3 Andreas Rosenwald,3 Christian Peschel,1 Ingo Ringshausen,1 Ulrich Keller1
1III Medical Department, 2Nuclear Medicine Department, Technische Universität München, Munich, Germany; 3Institute of Pathology, University of Würzburg, Würzburg, Germany
Abstract: Anaplastic large cell lymphoma (ALCL) is a common subtype of the heterogeneous group of peripheral T-cell lymphomas, which is characterized by large pleomorphic cells with strong expression of CD30. Translocations involving ALK, the anaplastic lymphoma kinase gene, are associated with a favorable clinical outcome. Such ALK-positive ALCLs are usually responsive to a multidrug chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). However, there is no general consensus on the optimal therapy for relapsed or refractory ALCL. We report the case of a 24-year-old male suffering from ALK-positive ALCL with an uncommon manifestation of only extranodal disease in the gastric cardia region that showed primary refractoriness to standard CHOP chemotherapy. A combination therapy consisting of the anti-CD30 drug conjugate, brentuximab vedotin, and classical lymphoma salvage regimen DHAP (cisplatin, high-dose cytarabine and dexamethasone) was administered. Following two treatment cycles in 21-day intervals, the lymphoma showed considerable regression based on imaging diagnostics and no evidence of vital lymphoma in a subsequent biopsy. We did not observe any increase in toxicity; in particular, polyneuropathy and febrile neutropenia were not observed. In summary, we report that the antibody-drug conjugate brentuximab vedotin and a classical regimen used for aggressive lymphoma, DHAP, could be combined as salvage therapy in a case of refractory ALK-positive ALCL. Phase I/II studies will be required for safety and efficacy analysis.
Keywords: anaplastic large cell lymphoma (ALCL), refractory/relapsed lymphoma, anti-CD30 drug conjugate, DHAP, combined therapy
Introduction
The primary systemic type of anaplastic large cell lymphoma (ALCL) is a common subtype of the heterogeneous group of peripheral T-cell lymphomas (PTCL). PTCL together account for less than 15% of all non-Hodgkin lymphomas in adults.1 The ALK-positive subtype of ALCLs is defined by different translocations involving the anaplastic lymphoma kinase (ALK) gene on chromosome 2 that result in an overexpression of a constitutively active kinase. Such ALK-positive ALCLs proved to have a more favorable outcome compared to ALK-negative lymphomas.2 ALCLs show bimodal age distribution, peaking in early and late adulthood, with the median age at the time of diagnosis being much lower in patients with ALK-positive ALCL (34 years versus 58 years in ALK-negative ALCL).3 Patients typically present with painless lymphadenopathy with concomitant B-symptoms. Extranodal disease manifestations are most common in skin, bone, lung, and liver. The neoplastic cells characteristically show strong expression of CD30 and cytotoxicity-associated antigens, while the expression of pan-T-cell antigens is often lost.4 Clinically, ALK-positive ALCLs typically show an aggressive course but are usually responsive to standard CHOP or CHOP-like therapy, with the most important prognostic factor being the International Prognostic Index (IPI) at the time of diagnosis.2 However, there is no general consensus on the optimal therapy for relapsed or refractory ALCL.5 Here, we report on a combination therapy with cisplatin, high-dose cytarabine, and dexamethasone (DHAP) and brentuximab vedotin, a CD30-directed antibody linked to the antitubulin agent monomethyl auristatin E,6,7 in a young patient with primarily refractory ALK-positive ALCL.
Case presentation
A 24-year-old male was admitted to a primary care hospital because of persistent dyspepsia, epigastric pain, and weight loss. A gastroscopy revealed an exophytic, ulcerating tumor in the cardia region and proximal corpus of the stomach with extension into the distal esophagus. Mucosal biopsies showed infiltration by an ALK-positive, CD20-negative large cell lymphoma accompanied by chronic Helicobacter pylori-negative antrum gastritis. A subsequent computed tomography (CT) scan and bone marrow biopsy showed exclusive involvement of the stomach. The IPI score was 0. Standard chemotherapy with CHOP was initiated. Under such anthracycline-based chemotherapy, patients with ALK-positive ALCL generally show a favorable outcome with a 5-year overall survival ranging from 70% to 93%.8,9 In this particular case, following three cycles of the CHOP regimen, a CT scan demonstrated partial remission. However, following cycle five, a CT scan of the chest and upper abdomen was performed because of productive coughing, which showed local tumor progression. The CHOP regimen was discontinued and the patient was subsequently transferred to our hospital.
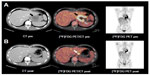
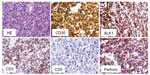
On admission the patient presented in good general condition but was suffering from worsening dyspepsia, shoulder pain, and night sweats. Physical examination was unremarkable; blood count and laboratory values were – apart from mild anemia (hemoglobin 13.7 g/dL) – within normal limits. [18F]Fluorodeoxyglucose positron emission tomography (PET)-CT revealed intensive tracer uptake by the known lymphoma manifestation in the cardia region, with a tumor size of 60 × 60 × 65 mm. Two additional nodules cranial to the primary tumor with enhanced glucose uptake were considered as affected lymph nodes (Figure 1A). Other manifestations were not identified. A second endoscopic mucosal biopsy showed infiltration by highly-proliferating lymphoid cells, staining positive for CD30 and CD3 with nuclear and cytoplasmic expression of ALK and negativity for CD19, CD79a, and CD38 (Figure 2), again showing ALK-positive large cell lymphoma, Ann Arbor stage IIE, age-adjusted IPI low risk. Based on the immunohistochemical features and the refractoriness to CHOP, ALK-positive large B-cell lymphoma (LBCL), which is usually characterized by CD20 negativity and sometimes stains positive for CD30,10 was also considered, and the biopsy was sent to a national reference center for hematopathology.
Considering the primary refractoriness towards standard CHOP treatment and the possibility of ALK-positive LBCL, which is associated with a poor prognosis,10 we discussed with the patient an individual treatment plan, consisting of the anti-CD30 drug conjugate, brentuximab vedotin and the classical lymphoma salvage regimen DHAP11 (brentuximab vedotin 1.2 mg/kg [day 0], cisplatin 70 mg/m2 [day 1] over a period of 22 hours, cytarabine [2 × 2,000 mg/m2 on day 2] and dexamethasone [40 mg on days 2–5]) (Figure 3). Standard supportive care was administered. For primary prophylaxis of prolonged neutropenia, pegfilgrastim 6 mg was administered on day 4. Because of the experimental chemotherapeutic design, dosing of the first cycle was reduced to 75%. The treatment was very well tolerated by the patient. Erosion bleedings related to tumor lysis were not observed. The shoulder pain and night sweats rapidly improved under therapy. Leukapheresis for autologous peripheral blood stem cells was performed following cycle one. A short-term interim staging by CT scan showed partial tumor regression. Reference pathology finally confirmed the diagnosis of ALCL against ALK-positive LBCL (Figure 2). The second cycle brentuximab vedotin/DHAP was administered without complications with 100% dose levels as stated above. The patient denied sensory or motor polyneuropathy; focal neurological deficiencies were not observed. Serum analysis showed normal liver and renal function tests. On the scale for Common Terminology Criteria for Adverse Events (CTCAE), hematological toxicity was limited to the expected grade 2–3 neutropenia and thrombocytopenia. No infections occurred during the course of chemotherapy. A second PET-CT scan after cycle two showed further tumor regression, both morphologically and with regards to glucose uptake (Figure 1B). A discrete swelling of the gastric wall with enhanced glucose metabolism remained. Biopsy and immunohistological analysis revealed inflammation without evidence of lymphoma. The presumably affected lymph nodes cranial to the primary tumor were no longer detectable. There was no evidence of any new lesions. The treatment response was considered as complete remission. Three weeks later, the treatment was continued with consolidation high-dose chemotherapy using the BEAM protocol (bis-chloroethylnitrosourea [BCNU], etoposide, AraC, melphalan)12 and autologous peripheral blood hematopoietic stem cell transplantation (HSCT). Considering the primary refractoriness to CHOP and stage II disease, consolidating involved-field radiotherapy was applied. At the time of submission of this manuscript, the patient had just finished radiotherapy and was doing very well without any clinical signs of relapse.
Discussion
There is no general consensus regarding the optimal treatment regimen in patients with refractory or relapsed PTCL. Such patients are generally treated with combination chemotherapy regimens in an attempt to achieve a remission, followed by consolidating high dose chemotherapy and autologous HSCT, or allogeneic HSCT in medically fit individuals. Consolidation therapy with autologous HSCT is a highly promising option only in patients with ALK-positive ALCL,13,14 with a complete remission at the time of transplantation and lack of extranodal disease being important predictors for a favorable outcome.15 Recently, novel agents for the treatment of refractory or relapsed PTCL have emerged, such as brentuximab vedotin, an anti-CD30 antibody-drug conjugate. In a Phase I clinical trial involving 45 patients with relapsed or refractory CD30+ hematopoietic neoplasms that were treated with brentuximab vedotin, 17 patients showed an objective response, and stable disease was reported in 19 patients.16 In a Phase II multicenter study, 58 patients with relapsed or refractory ALCL were treated with brentuximab vedotin (1.8 mg/kg every 3 weeks for up to 16 cycles). The overall response rate was 86% with an updated median progression-free survival of 14.6 months.17,18 As reported previously, most common adverse events were peripheral sensory neuropathy, nausea, fatigue, pyrexia, diarrhea, rash, constipation, and neutropenia. On the basis of these data, brentuximab vedotin was approved by the US Food and Drug Administration and the European Medicines Agency for monotherapy of ALCL after failure of at least one prior multiagent chemotherapy regimen. For a more detailed discussion of the most recent clinical studies on brentuximab vedotin in the treatment of ALCL and Hodgkin lymphoma, we refer to a recent review by Chen et al.5 Preliminary studies also showed effectiveness of brentuximab vedotin in CD30+ non-ALCL PTCL.5,19,20 Numerous clinical trials are now ongoing to evaluate the clinical benefit of combination of brentuximab vedotin with classical chemotherapy agents. Table 1 gives an overview on already active trials that investigate the combination of brentuximab vedotin and chemotherapy in adult patients with CD30+ ALCL or Hodgkin lymphoma. First studies have proven the feasibility of such an approach in patients with Hodgkin lymphoma.21–23 Preliminary data from an ongoing Phase I clinical study showed substantial antitumor activity and manageable toxicity with the combination of brentuximab vedotin and CHP (CHOP without vincristine) in patients with newly-diagnosed ALCL or other CD30+ mature T-cell/natural killer-cell lymphomas.24
Here, we report that the antibody-drug conjugate brentuximab vedotin and a multidrug chemotherapy regimen used for the treatment of aggressive lymphoma, DHAP, can be efficiently and safely combined as salvage treatment prior to autologous stem cell transplantation in a case of refractory ALK-positive ALCL. We did not observe an overt increase in toxicity; in particular, polyneuropathy and febrile neutropenia were not observed. Because of the experimental character of this treatment, brentuximab vedotin has been limited to 1.2 mg/kg. Such combination therapy might improve the outcome in patients with ALK-positive ALCL that do not meet favorable prerequisites for consolidating autologous HSCT. Furthermore, in some cases, the differentiation of ALCL from the anaplastic type diffuse LBCL or other lymphoid neoplasms of T or null cell origin can be challenging. In patients with arguable histological findings and thus uncertain prognosis, a monotherapy with brentuximab vedotin seems not sufficient, and such patients might benefit from the combination of a multiagent chemotherapy. In consideration of the encouraging data from preliminary studies of combination therapies, an individual treatment plan in such patients could be considered. In general, combined therapy with the anti-CD30 drug conjugate brentuximab vedotin with a classical chemotherapy backbone may be a promising approach in the future treatment of CD30-expressing lymphomas. Phase I/II trials for safety and efficacy assessment will be required.
Disclosure
The authors report no conflicts of interest in this work.
References
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. | |
Savage KJ, Harris NL, Vose JM, et al; International Peripheral T-Cell Lymphoma Project. ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. | |
Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. | |
Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87. | |
Chen X, Soma LA, Fromm JR. Targeted therapy for Hodgkin lymphoma and systemic anaplastic large cell lymphoma: focus on brentuximab vedotin. Onco Targets Ther. 2013;7:45–56. | |
Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–637. | |
Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–897. | |
Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999;93(8):2697–2706. | |
Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93(11):3913–3921. | |
Reichard KK, McKenna RW, Kroft SH. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol. 2007;20(3):310–319. | |
Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988;71(1):117–122. | |
Gaspard MH, Maraninchi D, Stoppa AM, et al. Intensive chemotherapy with high doses of BCNU, etoposide, cytosine arabinoside, and melphalan (BEAM) followed by autologous bone marrow transplantation: toxicity and antitumor activity in 26 patients with poor-risk malignancies. Cancer Chemother Pharmacol. 1988;22(3):256–262. | |
Rodriguez J, Caballero MD, Gutiérrez A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003;14(12):1768–1775. | |
Jagasia M, Morgan D, Goodman S, et al. Histology impacts the outcome of peripheral T-cell lymphomas after high dose chemotherapy and stem cell transplant. Leuk Lymphoma. 2004;45(11):2261–2267. | |
Fanin R, Ruiz de Elvira MC, Sperotto A, Baccarani M, Goldstone A. Autologous stem cell transplantation for T and null cell CD30-positive anaplastic large cell lymphoma: analysis of 64 adult and paediatric cases reported to the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1999;23(5):437–442. | |
Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. | |
Pro B, Advani RH, Brice P, et al. Three-year survival results from an ongoing phase 2 study of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2013;122(21):1809. | |
Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. | |
Duvic M, Tetzlaff M, Clos AL, Gangar P, Talpur R. Results of a phase II trial of brentuximab vedotin (SGN-35) for CD30+ cutaneous T-cell lymphomas and lymphoproliferative disorders. Abstract presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA. Blood. 2012;120(21): Abstract 3688. | |
Jacobsen ED, Advani RH, Oki Y, et al. A phase 2 Study of brentuximab vedotin in patients with relapsed or refractory CD30-positive non-Hodgkin lymphomas: interim results. Abstract presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA. Blood. 2012;120(21): Abstract 2746. | |
Eichenauer DA, Plütschow A, Kreissl S, et al. Targeted Beacopp variants In patients with newly diagnosed advanced stage classical Hodgkin lymphoma: interim results of a randomized phase II study. Abstract presented at: 55th ASH Annual Meeting and Exposition; December 7–10, 2012; New Orleans, LA. Blood. 2013;122(21): Abstract 4344. | |
Moskowitz A, Schoder H, Gerecitano JF, et al. FDG-PET adapted sequential therapy with brentuximab vedotin and augmented ICE followed by autologous stem cell transplant for relapsed and refractory Hodgkin lymphoma. Abstract presented at: 55th ASH Annual Meeting and Exposition; December 7–10, 2012; New Orleans, LA. Blood. 2013;122(21): Abstract 2099. | |
Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–1356. | |
Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin administered before, during, and after multi-agent chemotherapy in patients (pts) with newly-diagnosed CD30+ mature T- and NK-cell lymphomas. Abstract presented at: 55th ASH Annual Meeting and Exposition; December 7–10, 2012; New Orleans, LA. Blood. 2013;122(21): Abstract 4386. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.