Back to Journals » Drug Design, Development and Therapy » Volume 9
Colon-targeted delivery of piceatannol enhances anti-colitic effects of the natural product: potential molecular mechanisms for therapeutic enhancement
Authors Yum S, Jeong S, Lee S, Nam J, Kim W, Yoo J , Kim M, Lee BL, Jung Y
Received 18 May 2015
Accepted for publication 18 June 2015
Published 4 August 2015 Volume 2015:9 Pages 4247—4258
DOI https://doi.org/10.2147/DDDT.S88670
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shu-Feng Zhou
Soohwan Yum, Seongkeun Jeong, Sunyoung Lee, Joon Nam, Wooseong Kim, Jin-Wook Yoo, Min-Soo Kim, Bok Luel Lee, Yunjin Jung
College of Pharmacy, Pusan National University, Busan, Republic of Korea
Abstract: Piceatannol (PCT), an anti-colitic natural product, undergoes extensive Phase II hepatic metabolism, resulting in very low bioavailability. We investigated whether colon-targeted delivery of PCT could enhance anti-colitic effects and how therapeutic enhancement occurred at the molecular level. Molecular effects of PCT were examined in human colon carcinoma cells and inflamed colons. The anti-colitic effects of PCT in a colon-targeted capsule (colon-targeted PCT) were compared with PCT in a gelatin capsule (conventional PCT) in a trinitrobenzene sulfonic acid-induced rat colitis model. Colon-targeted PCT elicited greatly enhanced recovery of the colonic inflammation. In HCT116 cells, PCT inhibited nuclear factor kappaB while activating anti-colitic transcription factors, nuclear factor-erythroid 2 (NF-E2) p45-related factor 2, and hypoxia-inducible factor-1. Colon-targeted PCT, but not conventional PCT, modulated production of the target gene products of the transcription factors in the inflamed colonic tissues. Rectal administration of PCT, which simulates the therapeutic action of colon-targeted PCT, also ameliorated rat colitis and reproduced the molecular effects in the inflamed colonic tissues. Colon-targeted delivery increased therapeutic efficacy of PCT against colitis, likely resulting from multitargeted effects exerted by colon-targeted PCT. The drug delivery technique may be useful for therapeutic optimization of anti-colitic lead compounds including natural products.
Keywords: piceatannol, colitis, colon-targeted delivery, multitarget, polypharmacology
Introduction
Inflammatory bowel disease (IBD), such as ulcerative colitis and Crohn’s disease, is a chronic relapsing idiopathic disease.1 Conventional therapies for ulcerative colitis and Crohn’s disease include aminosalicylates, corticosteroids, thiopurines, methotrexate, and antitumor necrosis factor agents.2,3 Most patients require lifelong medication yet experience frequent relapses due to therapeutic imperfectness and possible unpleasant adverse effects of the conventional therapies.4 Therefore, development of a new drug with improved therapeutic outcome is warranted.
Some transcription factors such as nuclear factor kappaB (NFκB), nuclear factor-erythroid 2 (NF-E2) p45-related factor 2 (Nrf2), and hypoxia-inducible factor-1 (HIF-1) have pathophysiological roles in the progression of IBD.5–7 In the inflammatory state, NFκB, a central regulator involved in immunity and inflammation, is activated, which is believed to recruit immune cells and induce inflammatory reactions in the large intestine.5 On the other hand, HIF-1α, a master regulator of oxygen homeostasis, and Nrf2, a regulator in cytoprotection against inflammation as well as oxidative and electrophilic stresses, are likely to exert protective roles against intestinal inflammation.7–9 In fact, functional or genetic loss of the transcription factors through intestinal epithelium-targeted expression of mutant HIF-1α or Nrf2 knockout increases susceptibility to murine experimental colitis.6,7 Consistent with these findings, chemical or biological activators of the transcription factors have beneficial effects on experimental colitis.8,10
Piceatannol (PCT) is a trans-3,4,3′,5′-tetrahydroxystilbene first isolated from the seeds of Euphorbia lagascae.11 At the molecular level, PCT modulates the activity of some transcription factors including NFκB,12,13 Nrf2,14 and HIF-1α.15 These molecular effects of PCT suggest that PCT may be beneficial in the treatment of gut inflammation. In fact, PCT shows a positive effect on experimental colitis upon oral administration at a dose of 10 mg/kg.16,17 However, recent reports dealing with pharmacokinetics of PCT analogs suggest that the pharmacological effect of PCT could be enhanced by improving its therapeutic bioavailability. PCT administered orally extensively undergoes Phase II hepatic metabolism such as glucuronidation and sulfation, resulting in very low bioavailability.18
Colon-specific drug delivery is an effective strategy to increase the therapeutic bioavailability of a drug at the large intestine, thereby conferring therapeutic advantages for treatment of colonic diseases such as IBD. Therefore, this drug delivery technique is applied for anti-inflammatory drugs such as 5-aminosalicylic acid and glucocorticoids.19 In this study, colon-targeted PCT was prepared in our laboratory and administered orally to colitic rats; its anti-colitic activity was then evaluated and compared with conventional PCT. In addition, potential molecular mechanisms for the therapeutic effects of colon-targeted PCT were explored.
Materials and methods
Chemicals and animals
PCT was obtained from Tokyo Chemical Industry (Tokyo, Japan). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma Chemical Co. (Perth, Australia). All other chemicals were reagent-grade, commercially available products. Ten-week-old male male Sprague Dawley rats (Samtako Bio Korea, Osan-si, Republic of Korea) were housed in the university animal facility with controlled temperature, humidity, and dark/light cycle. The animal protocol used in this study has been reviewed and approved by the Pusan National University-Institutional Animal Care and Use Committee based on their ethical procedures and scientific care.
Cell culture and transient transfection
Human colon carcinoma cells HCT116 and murine macrophages RAW 264.7 cells were grown in Dulbecco’s Modified Eagle’s Medium (HyClone, South Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone) and penicillin/streptomycin (HyClone). Cell transfection was performed as described previously.20 Cells were lysed for 24 hours (for Nrf2-responsive luciferase) or 6 hours (for NFκB-responsive luciferase) after treatment with reagents at the indicated concentrations in the figure legends and luciferase activities were measured and normalized to CMV Renilla luciferase activities using a Dual Luciferase reporter assay kit (Promega Corporation, Fitchburg, WI, USA). Nrf2 knockdown (shHCT116) and control (scHCT116) HCT116 cell lines were established as reported previously.21
Immunoblot analysis
Cells were lysed and nuclear and cytosolic extracts were prepared as described22 and tissue total lysates were prepared using the inflamed distal colon.20 Protein concentrations in the lysates were determined by the bicinchoninic acid assay. Cell or tissue extracts were subjected to Western blot analysis.20 Nrf2, p65, and HIF-1α were detected in nuclear extracts (30–40 μg) using monoclonal anti-Nrf2 (Abcam, Cambridge, MA, USA), p65 (Santa Cruz Biotechnology Inc., Dallas, TX, USA), and HIF-1α (BD Biosciences, San Jose, CA, USA) antibodies, and hemeoxygenase-1 (HO-1) protein was detected in whole cell (30–40 μg) or tissue lysates (30–40 μg) using monoclonal anti-HO-1, COX-2, and iNOS antibodies (Santa Cruz Biotechnology). Secondary antibodies (Santa Cruz Biotechnology) for each primary antibody were used at a dilution of 1:2,000. SuperSignal chemiluminescence substrate (Pierce, Rockford, IL, USA) was used for visualizing signals. Experiments were performed in duplicate and normalized with antibodies to topoisomerase II (Santa Cruz Biotechnology) for transcription factors and to α-tubulin (Santa Cruz Biotechnology) for HO-1, COX-2, and iNOS.
Induction and evaluation of TNBS-induced colitis
Colonic inflammation was induced by intracolonic instillation of TNBS as described previously.20 A gross colonic damage score was calculated according to the criteria set forth previously.23,24 The modified scoring system is shown in Figure S1. The colonic damage score was assessed by four observers blinded to the treatment. Myeloperoxidase activity, an inflammatory indicator of colitis, was measured in the distal colon (5 cm) as described previously.25 To assess recovery of colonic injury, colonic tissue sections were subjected to hematoxylin-eosin (H&E) staining as described previously.26
Preparation of colon-targeted PCT and oral and rectal administration
A colon-specific polymer was prepared in our laboratory as described previously.27 Rodent capsules (Φ 2.65×4.3 mm, Qualicaps, Yamatokoriyama, Japan) were trimmed down to its half size and filled with PCT. The capsules were coated with the colon-specific polymer and EUDRAGIT® S 100 (Röhm Pharma GmbH, Darmstadt, Germany).26 Rats, starving for 24 hours except for water, were anesthetized with diethyl ether. A colon-targeted capsule filled with PCT (10 mg/kg, colon-targeted PCT) or uncoated capsule filled with PCT (10 mg/kg, conventional PCT) was administered to rats by gavage 3 days after induction of colitis. For intracolonic treatment with PCT, a rubber catheter (OD, 2 mm) was inserted rectally into the colon of rats lightly anesthetized with diethyl ether and PCT (100 μM in 0.5 mL phosphate-buffered saline [PBS]) or PBS (0.5 mL) was instilled into the colon via the rubber cannula 3 days after induction of colitis. Rats were medicated once a day and were sacrificed 6 days later.
ELISA of CINC-3, VEGF, and IL-8
The inflamed distal colon was removed, mixed with pH 6 potassium phosphate buffer, homogenized, and centrifuged at 2,500× g (4°C for 3 minutes) and 10,000× g (4°C for 10 minutes). Cytokine-induced neutrophil chemoattractant-3 (CINC-3) and vascular endothelial growth factor (VEGF) were measured in the supernatants using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Interleukin-8 (IL-8) in the cell culture supernatants were measured using an ELISA kit (R&D Systems) according to manufacturer’s instruction.
Data analysis
Results are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey’s HSD test was used for testing the differences between data. Differences with P<0.05 were considered significant. PASW statistics 18 (IBM SPSS, Chicago, IL, USA) was used for the statistical analysis.
Results
Colon-targeted delivery of PCT substantially enhances the anti-colitic activity in TNBS-induced rat colitis
PCT elicits an anti-inflammatory effect in experimental colitis models.16,17 Since colon-specific drug delivery tends to enhance therapeutic effect of the drug against colonic diseases such as IBD,19 PCT was prepared to be colon-specific by loading it in a colon-targeted capsule (colon-targeted PCT) that was then administered orally to TNBS-induced colitis rats. As a control, PCT in a gelatin capsule (conventional PCT) was subjected to the same experiments. As shown in Figure 1A, colon-targeted PCT healed the injury induced by TNBS much more effectively than conventional PCT. Photos of the colonic tissues are shown in Figure S2. Consistent with CDS results, colon-targeted PCT lowered myeloperoxidase activity to a greater extent than conventional PCT (Figure 1B). H&E staining was also performed to examine injury healing. Colon-targeted PCT induced greater recovery of the inflamed colons than conventional PCT (Figure S3).
PCT modulates the activities of transcription factors involved in the progression of gut inflammation
To investigate the molecular mechanisms underlying the anti-colitic activity of colon-targeted PCT, we examined whether PCT could modulate the activities of transcription factors involved in regulation of gut inflammation, HIF-1, Nrf2, and NFκB, in human colon carcinoma cells. While activation of Nrf2 and HIF-1α is beneficial to experimental colitis,8,10 NFκB inhibition ameliorates intestinal inflammation.5 PCT activation of HIF-1 in colon carcinoma HCT116 cells has been demonstrated,15 and PCT activation of an ulcer healing pathway HIF-1–VEGF is shown in Figure S4. We examined whether PCT modulated Nrf2 and NFκB activity in colon carcinoma cells. HCT116 cells were transfected with luciferase plasmids responsive to each transcription factor and then treated with PCT. As shown in Figure 2A, PCT induced Nrf2-dependent luciferase activities but inhibited tumor necrosis factor (TNF) induction of NFκB-dependent luciferase activity. To confirm PCT modulation of the transcription factor activities, the nuclear accumulation of the transcription factors – representing transcription factor activation – was monitored after treatment with PCT. As shown in Figure 2B, in parallel with the results of luciferase experiments, PCT promoted nuclear accumulation of Nrf2 while preventing nuclear accumulation of p65 by TNF-α. To assess whether PCT modulation of the transcription factor activities was relevant to the anti-colitic effect of the natural product, induction of target gene products was monitored following treatment with PCT in cells. The target gene products were HO-1 (a target gene of Nrf2 involved in anti-inflammatory and cytoprotective activity) and IL-8, COX-2 and iNOS (target genes of NFκB involved in gut inflammation). Simultaneously, to verify that the induction of the gene products was dependent on the corresponding transcription factors, cells stably transfected with Nrf2 shRNA were incubated with PCT, and the levels of the target gene products were monitored. As shown in Figure 2C–E, PCT promoted induction of HO-1, which was diminished substantially in cells transfected with Nrf2 shRNA (Figure 2C), and suppressed TNF secretion of IL-8 in HCT116 cells (Figure 2D) and lipopolysaccharide (LPS) induction of COX-2 and iNOS in RAW264.7 cells (Figure 2E). TNF induction of IL-8 secretion and LPS induction of COX-2 and iNOS in each cell line are dependent on NFκB.26 Finally, to examine whether the cellular effects were relevant to the anti-colitic effects of colon-targeted PCT, the target gene products of the transcription factors were monitored in the inflamed colonic tissues after the oral administration of colon-targeted PCT or conventional PCT. As shown in Figure 3A–C, colon-targeted PCT increased the level of VEGF (Figure 3A) while decreasing the levels of CINC-3 (Figure 3B), COX-2 and iNOS (Figure 3C). In contrast, conventional PCT did not significantly modulate the levels of the target genes in the inflamed colonic tissue. Compared with TNBS control, colon-targeted PCT marginally elevated HO-1 level (Figure 3D).
Intracolonic treatment with PCT elicits anti-colitic effects and reproduces the molecular effects in the inflamed colon
PCT delivered specifically to the large intestine (colon-targeted PCT) should act locally at the inflamed colon. To examine whether colon-targeted PCT exerted anti-colitic effects via local action, PCT (100 μM in 0.5 mL PBS) was administered rectally to rats with TNBS-induced colitis once a day for 6 days, beginning 3 days after inflammation induction. The concentration of PCT was decided based on previous papers demonstrating that oral administration of colon-targeted celecoxib or quercetin at the same dose (10 mg/kg) renders colonic concentrations of the compounds of more than 100 μM.28,29 As shown in Figure 4A and B, rectally administered PCT improved the indicators of colonic injury, CDS (Figure 4A), myeloperoxidase (Figure 4B) and H&E staining (Figure S5). To verify that the therapeutic effect of rectal PCT occurred via the same molecular mechanisms, the target gene products were monitored in the inflamed colon. Like colon-targeted PCT, rectal PCT attenuated the levels of NFκB target gene products, CINC-3 (Figure 4C), COX-2, and iNOS-1 (Figure 4D), while elevating the levels of an HIF-1 target gene product, VEGF (Figure 4E). The level of HO-1 did not increase compared with the TNBS control (data not shown). This in vivo outcome for HO-1 induction was in contrast with cellular effects of PCT. Since caffeic acid phenethyl ester, a catechol-bearing compound, induces Nrf2 activation in cells but requires oxidation (inflammation) of the catechol moiety for effective activation of Nrf2 in the colonic tissues,20 PCT, which also has a catechol moiety, may act as caffeic acid phenethyl ester. To test this, PCT was administered rectally the third day after inflammation induction, when the colonic inflammation was still in its prime, and HO-1 was detected in the inflamed colonic tissue 4 and 8 hours after PCT administration. As shown in Figure 4F, consistent with the previous observation,20 PCT induction of HO-1 was clear.
Discussion
In this study, the anti-inflammatory activity of colon-targeted PCT was evaluated and compared with conventional PCT. In addition, potential molecular mechanisms for amelioration of rat colitis by colon-targeted PCT were investigated. Our data demonstrate that colon-targeted PCT was much more effective than conventional PCT in mitigating rat colitis, and colon-targeted PCT may enhance the therapeutic effects by intervening in multiple pathways through modulation of activities of transcription factors implicated in IBD pathogenesis.
Consistent with our hypothesis that colon-targeted PCT can improve the therapeutic activity of conventional PCT against experimental colitis, colon-targeted PCT showed enhanced anti-inflammatory effects in the rat colitis compared with conventional PCT. We suggest that the therapeutic superiority is due to increased therapeutic concentration at the inflamed site by colonic delivery of PCT, which may change the anti-colitic pharmacology of PCT. This argument is based on the following observations: PCT modulated activities of transcription factors, Nrf2, HIF-1, and NFκB in the concentration range of 10–50 μM, which could be achieved with colon-targeted PCT but not conventional PCT.28,29 Oral administration of conventional PCT (10 mg/kg) renders the plasma concentration of PCT to reach 1 μM at most.18 Indeed, in contrast with conventional PCT, colon-targeted PCT significantly modulated the levels of the target gene products in the inflamed colonic tissues, which was reproduced by rectal administration of PCT, simulating a therapeutic situation of colon-targeted PCT. Thus, colon-targeted PCT may act as a multitargeted therapeutic agent against experimental colitis; colon-targeted PCT not only inhibited NFκB to suppress inflammatory responses but also activated HIF-1 and Nrf2 to enhance the intestinal barrier function and ulcer healing activity.
Our data showing that conventional PCT (10 mg/kg) had no significant anti-colitic effects, which are not consistent with previous papers demonstrating anti-colitic effects of PCT in dextran sulfate sodium-induced colitis.16,17 Although we do not have a good explanation on this therapeutic discrepancy, it may be due to species difference. Dextran sulfate sodium-induced mouse colitis model was used to investigate anti-colitic effects of PCT while TNBS-induced rat colitis model was employed in this study. The metabolisms that inactivate PCT may take place in the animals at different rates, probably resulting in variation in therapeutic activity of PCT.
In line with a previous paper demonstrating that caffeic acid phenethyl ester, a catecholic natural product, effectively activates the Nrf2–HO-1 pathway in the inflamed colon but not in the normal colon,20 HO-1 induction by PCT did not occur in the colon where inflammation was alleviated. Like caffeic acid phenethyl ester, oxidation of the catechol in PCT to its electrophilic quinone, a typical activator of Nrf2, may occur more efficiently in inflammation. In the resolved phase of inflammation, PCT may not affect Nrf2–HO-1 pathway as much as in the early phase of inflammation. Although we did not examine inflammation-dependent effects on PCT modulation of other transcription factors, PCT effects on HIF-1 and NFκB may also be subject to such change. This argument is based on recent papers demonstrating that oxidation of the catechol in PCT is involved in PCT inhibition of NFκB,13 while the catechol moiety is required for activation of HIF-1.15 It would be intriguing to examine the inflammation-dependent change in the PCT pharmacology, which should affect the therapeutic outcome against colitis.
Generally, multitargeted therapy improves efficacy compared with mono-targeted therapy.30 Simultaneously, it is more likely that a multitargeted drug, a promiscuous ligand, acts on off-target(s) leading to side effects.30 Thus, it is important to consider the target selectivity of the multitargeted drug. To minimize the risk of side effects, the target selectivity can be improved by manipulating the pharmacodynamic (drug–target interaction) or pharmacokinetic properties of the drug. Colon-specific drug delivery modulates pharmacokinetics of a drug by limiting the distribution of the drug at the target site, consequently leading to reduced systemic side effects.19 In this sense, colon-targeted PCT should have toxicological advantages over conventional PCT as well as therapeutic superiority.
In conclusion, PCT has the potential to be developed as a multitargeted drug for treatment of IBD, and adoption of colon-specific drug delivery should confer therapeutic and toxicological advantages on PCT, facilitating anti-IBD drug development from the lead, PCT.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2009-0083538).
Disclosure
The authors report no conflicts of interest in this work.
References
Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62(11):1653–1664. | ||
Ardizzone S, Porro GB. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252(6):475–496. | ||
Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20(7):1082–1096. | ||
Feagan BG. Maintenance therapy for inflammatory bowel disease. Am J Gastroenterol. 2003;98(12 Suppl):S6–S17. | ||
Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263(6):591–596. | ||
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580–11584. | ||
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114(8):1098–1106. | ||
Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1–2):12–23. | ||
Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134(1):145–155. | ||
Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134(1):156–165. | ||
Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat Res. 2011. | ||
Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169(11):6490–6497. | ||
Son PS, Park SA, Na HK, Jue DM, Kim S, Surh YJ. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-{kappa}B activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKK{beta} as a potential target. Carcinogenesis. 2010;31(8):1442–1449. | ||
Lee HH, Park SA, Almazari I, Kim EH, Na HK, Surh YJ. Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch Biochem Biophys. 2010;501(1):142–150. | ||
Yum S, Doh HJ, Hong S, et al. Piceatannol, a hydroxystilbene natural product, stabilizes HIF-1alpha protein by inhibiting HIF prolyl hydroxylase. Eur J Pharmacol. 2013;699(1–3):124–131. | ||
Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61(6):847–854. | ||
Kim YH, Kwon HS, Kim DH, et al. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2008;8(12):1695–1702. | ||
Roupe KA, Yanez JA, Teng XW, Davies NM. Pharmacokinetics of selected stilbenes: rhapontigenin, piceatannol and pinosylvin in rats. J Pharm Pharmacol. 2006;58(11):1443–1450. | ||
Jung Y, Kim YM. What should be considered on design of a colon-specific prodrug? Expert Opin Drug Deliv. 2010;7(2):245–258. | ||
Kim H, Kim W, Yum S, et al. Caffeic acid phenethyl ester activation of Nrf2 pathway is enhanced under oxidative state: structural analysis and potential as a pathologically targeted therapeutic agent in treatment of colonic inflammation. Free Radic Biol Med. 2013;65:552–562. | ||
Lee Y, Shin DH, Kim JH, et al. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur J Pharmacol. 2010;643(1):21–28. | ||
Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19(9):2499. | ||
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. | ||
Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Control Release. 2002;79(1–3):103–112. | ||
Son MW, Ko JI, Doh HM, et al. Protective effect of taurine on TNBS-induced inflammatory bowel disease in rats. Arch Pharm Res. 1998;21(5):531–536. | ||
Hong S, Yum S, Yoo HJ, et al. Colon-targeted cell-permeable NFkappaB inhibitory peptide is orally active against experimental colitis. Mol Pharm. 2012;9(5):1310–1319. | ||
Yamaoka T, Makita Y, Sasatani H, Kim SI, Kimura Y. Linear type azo-containing polyurethane as drug-coating material for colon-specific delivery: its properties, degradation behavior, and utilization for drug formulation. J Control Release. 2000;66(2–3):187–197. | ||
Lee Y, Kim J, Kim H, et al. N-succinylaspart-1-yl celecoxib is a potential colon-specific prodrug of celecoxib with improved therapeutic properties. J Pharm Sci. 2012. | ||
Kim H, Kong H, Choi B, et al. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res. 2005;22(9):1499–1509. | ||
Anighoro A, Bajorath J, Rastelli G. Polypharmacology: challenges and opportunities in drug discovery. J Med Chem. 2014. |
Supplementary materials
  | Figure S1 Modified scoring system. |
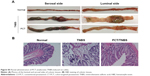
  | Figure S2 Photos of the luminal and serosal sides of colonic tissues. |
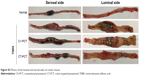
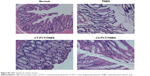  | Figure S3 H&E staining of colonic tissues. |
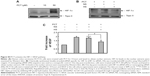  | Figure S4 PCT activates the HIF-1–VEGF pathway. |
Reference
Yum S, Doh HJ, Hong S, et al. Piceatannol, a hydroxystilbene natural product, stabilizes HIF-1alpha protein by inhibiting HIF prolyl hydroxylase. Eur J Pharmacol. 2013;699(1–3):124–131. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.