Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 8
Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins
Authors Di Pierro F , Bellone I, Rapacioli G, Putignano P
Received 8 December 2014
Accepted for publication 30 December 2014
Published 4 February 2015 Volume 2015:8 Pages 89—96
DOI https://doi.org/10.2147/DMSO.S78877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Francesco Di Pierro,1 Iaele Bellone,2 Giuliana Rapacioli,3 Pietro Putignano4
1Scientific Department, Velleja Research, Milan, Italy; 2ASL TO1, Turin, Italy; 3AIOR, Pontenure, Province of Piacenza, Italy; 4University Hospital San Gerardo, Monza, Italy
Background: Statin intolerance is a medical condition often leading patients to nonadherence to the prescribed therapy or to a relevant reduction of the statin dosage. Both situations determine a totally or partially uncontrolled lipid profile, and these conditions unquestionably increase the risk of cardiovascular events.
Methods: We enrolled hypercholesterolemic, type 2 diabetic patients complaining of intolerance to statins. Some of them had reduced the statin dose ‘until the disappearance of symptoms’; others had opted for treatment with ezetimibe; and yet others were not undergoing any treatment at all. All patients of the three groups were then given a fixed combination of berberine and silymarin (Berberol®), known from previous papers to be able to control both lipidic and glycemic profiles.
Results: The tested product both as a single therapy and as add-on therapy to low-dose statin or to ezetimibe reduced triglycerides, low-density lipoprotein cholesterol, fasting blood glucose, and glycosylated hemoglobin in a significant manner without inducing toxicity conditions that might be somehow ascribed to a statin-intolerant condition.
Conclusion: Our study demonstrates that use of Berberol®, administered as a single or add-on therapy in statin-intolerant subjects affected by diabetes and hypercholesterolemia is a safe and effective tool capable of improving the patients' lipidic and glycemic profiles.
Keywords: berberine, silymarin, Berberol®, ezetimibe, cholesterol, type 2 diabetes
Introduction
A high level of low-density lipoprotein cholesterol (LDL-C), especially if small and dense, is one of the main risk factors leading to cardiovascular diseases and death.1 Statins, thanks to their role in inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, are the chief drugs used worldwide to lower such a risk.2 Side effects of statins include muscle pain, fatigue, weakness, rhabdomyolysis (rarely), accompanied by an increase in creatine phosphokinase (CPK) and liver transaminase. Apart from rhabdomyolysis, adverse events may occur in 10% of all cases, especially when statins are given at high dosages, resulting in 30% treatment discontinuation.3 It goes without saying that use of a lower statin dosage may reduce the risk of statin-induced side effects, but at the same time often contributes to limiting the benefits on the lipid profile.4 Another possible strategy is to administer a different class of cholesterol-lowering drugs such as, for example, ezetimibe. In most case, however, its use does not result in a good lipidic target and has to be added to statins so that better clinical results can be obtained.5 In recent years, many nutraceutical ingredients, often of botanical origin, have been investigated in terms of their ability to control lipidic and/or glycemic profiles clinically. One of the most extensively investigated ingredients is the herbal alkaloid berberine. It has been demonstrated to be effective in patients with type 2 diabetes, in which it significantly decreases fasting and postprandial blood glucose and glycosylated hemoglobin (HbA1c) levels, with an effect similar to that of metformin,6 although it acts with somewhat different mechanisms including, for instance, increasing the expression of the insulin receptor.7 With regard to the lipidic profile, berberine upregulates the LDL-receptor expression, which results in total cholesterol and LDL-C reduction amounting to about 30% and 25%, respectively. This up-modulation occurs through a post-transcriptional mechanism that stabilizes the mRNA and makes berberine a cholesterol-lowering drug whose mechanism of action is different from that of statins.8 Although berberine acts as a glucose- and lipid-lowering agent, it remains rather defective in terms of oral bioavailability.9 In humans, this appears to be due to a P-glycoprotein (P-gp)–mediated gut extrusion process and massive biliary excretion.10,11 The amount of berberine capable of crossing enterocytes seems to be reduced by about 90% by P-gp, and this suggests that either use of a potential P-gp inhibitor or a chemical modification of berberine, allowing it to overcome P-gp antagonism, may enhance its poor oral bioavailability, thus increasing its clinical effectiveness.12,13 Among the potential P-gp inhibitors, silymarin from Silybum marianum, a herbal drug traditionally used as a liver protectant, could be considered a good candidate owing to its very poor oral bioavailability and high safety profile.14 The clinical efficacy of the fixed combination of berberine with silymarin (Berberol®) in improving lipidic and glycemic profiles was evaluated in some pilot clinical trials conducted in type 2 diabetic and/or hypercholesterolemic individuals with good results.15–18 It must be said, however, that these pilot trials enrolled patients who were tolerant to statins. Therefore, also in consideration of the safety profile of this fixed association, we decided to test it in type 2 diabetic, hypercholesterolemic patients who were intolerant to high-dosage statins as an add-on therapy to low-dose statin and as add-on therapy to ezetimibe, or alone in patients intolerant to any dose of statins.
Materials and methods
Study design
This 12-month, controlled pilot trial was conducted in the field of routine clinical practice, in accordance with the principles stated in the Declaration of Helsinki and consistent with Good Clinical Practice, as defined by the International Conference on Harmonization and in accordance with the ethical principles underlying the European Union Directive 2001/20/EC and the United States Code of Federal Regulations, Title 21, Part 50 (21cFR50).19 The protocol, informed consent, and privacy document were approved by the local ethical board. The study was carried out between October 2011 and March 2013. Suitable patients, identified from a review of the case notes and/or computerized clinic registers, were contacted by the investigators in person or by telephone. The study enrolled 45 patients diagnosed with type 2 diabetes and hypercholesterolemia and characterized by a clear intolerance to statins. All patients provided their written informed consent to participate in this study after a full explanation of the study had been given. All the enrolled participants completed the study.
Criteria
Inclusion criteria were: 1) informed consent and privacy forms signed and returned; 2) patients with a diagnosis of type 2 diabetes with hypercholesterolemia and statin intolerance; 3) age between 18 and 80 years; and 4) a negative pregnancy test for female patients. Exclusion criteria were: 1) refusal to sign the informed consent or privacy forms; 2) severe liver disorders and/or abnormal renal function (serum creatinine higher than 115 μmol/L); 3) severe heart dysfunction (class III or higher according to the New York Heart Association classification); 4) history of acute diabetes complications including ketoacidosis or hyperosmolar hyperglycemic nonketotic coma; 5) history of myocardial infarction or stroke; 6) malignancy; 7) psychiatric disease; 8) neuropathy; 9) severe infection; 10) breastfeeding, pregnancy, or planned pregnancy; 11) fasting plasma glucose with a value of 200 mg/dL or higher; 12) use of P-gp antagonists; and 13) alcohol or drug abuse.
Concomitant antidiabetic therapies
The glycemic control of all participants was suboptimal despite the prescription of a diet, physical exercise, and/or hypoglycemic drugs. On enrollment, hypoglycemic drugs were: metformin; gliclazide; repaglinide; metformin and sulfonylureas; metformin and glyburide; metformin and dipeptidyl peptidase-4 (DPP-IV) inhibitors; metformin plus pioglitazone; and metformin plus sulfonylurea and pioglitazone.
Lipidic control
The lipidic control of the participants was not optimal. Among the 45 individuals enrolled in the trial, 15 who were not being treated with hypolipidemic drugs on enrollment due to a recent diagnosis of hypercholesterolemia and statin intolerance served as controls; 15 were diagnosed with hypercholesterolemia and treated with ezetimibe (10 mg/day) due to statin intolerance diagnosed in the previous year; 15 were taking low doses of statins due to a diagnosed intolerance to high-dosage statins. Statins were: 1) simvastatin at 20 mg/day administered for 3–6 months before the enrollment then reduced to 10 mg/day; or 2) atorvastatin at 20 mg/day administered for 3 months before the enrollment then reduced to 10 mg/day; or 3) rosuvastatin at 10 mg/day administered for 3 months before the enrollment then reduced to 5 mg/day; or 4) fluvastatin at 40 mg/day administered for 3 months before the enrollment then reduced to 20 mg/day. When statins were reduced by 50% of the dosage, Berberol® was administered as add-on therapy.
Other concomitant therapies
Some of the patients were being treated with antihypertensive drugs and/or Aspirin Cardio, ticlopidine or warfarin. Some were under treatment with allopurinol, with drugs to control hypothyroidism and with tamsulosin. Patients were authorized to take the birth control pill, anti-inflammatory/analgesic and/or antibiotic and/or antifungal and/or beta-agonist (as inhalers) drugs in case of need.
Diagnosis of statin intolerance
The patients were considered intolerant if, following proper statin use, they showed a CPK increase that was three to ten times higher than the upper laboratory limit (ULL) and/or an elevation in transaminase values three to five times higher than the ULL and/or the onset of asthenia, myalgia, or rhabdomyolysis (although the last never occurred).
Study protocol and treatments
A scheme is presented in Figure 1. All participants were instructed to follow their usual hypocaloric, low glycemic index diet throughout the study. The controlled-energy diet (a daily caloric deficit of about 600 kcal) was based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) recommendations that contained 50% of calories from carbohydrates, 30% from fats (<7% saturated, up to 10% polyunsaturated, and up to 20% monounsaturated fats), and 20% from proteins, with a maximum cholesterol content of 300 mg/day, and 35 g/day of fiber. Standard diet advice was provided by a dietitian and/or specialist physician. The participants were also encouraged to keep on following their usual standardized physical activity (riding a stationary bike for 20–30 minutes, three to four times a week, or three to four 30-minute sessions of brisk walking in 1 week). After enrolling, all participants started treatment with the product formula (Berberol® one tablet every 12 hours, on an empty stomach). Treatment lasted 1 year. Blood analytical checks were performed on enrollment and 6 and 12 months later. All participants were instructed to record the onset of any adverse events in a personal daily document, with the specific indication of their characteristics (severity, duration, and possible cause–effect relationship with the drug administration), the number of missed tablets, and any changes in the diet, physical exercise, or weight.
  | Figure 1 Scheme of the study. |
Product formula
All the patients were treated as an adjunctive, or single, therapy with a nutraceutical association in tablets (Berberol®; PharmExtracta, Pontenure, Italy) containing 588 mg/tablet Berberis aristata extract titrated as 85% berberine, 105 mg/tablet S. marianum extract titrated as >60% flavonolignans. The Ministry of Health was notified about the product in 2010, in agreement with the Italian law number 169/2004 (Registration number: E10 40753Y). The product was registered as a food supplement with both active ingredients (standardized B. aristata and S. marianum extracts) belonging to the positive list of botanicals admitted as nutraceuticals, with all of its excipients being food grade.
Assessments
Before starting the study, all patients underwent an initial screening assessment that included medical history, physical examination, vital signs (blood pressure and heart rate), electrocardiogram, measurement of height and body weight, calculation of body mass index (BMI), assessment of fasting blood glucose (FG), HbA1c, total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). After 6 and 12 months of treatment, the following parameters were evaluated: TC, LDL-C, HDL-C, TG, FG, and HbA1c. These were chosen to be the primary endpoints. On enrollment and after 6 and 12 months, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and CPK were also verified to evaluate possible worsening of the statin intolerance conditions.
Safety measures
Treatment tolerability was assessed with accurate interviews of the patients by the investigators and comparisons of clinical and laboratory values with the baseline levels. Safety monitoring included physical examination, vital sign assessment, weight, electrocardiogram, and adverse event recording. Treatment tolerability, compliance, and side effects were chosen to be secondary endpoints.
Statistical analysis
The Wilcoxon signed-rank test was used for all the longitudinal comparison tests, that is, between individuals of the same treatment group at different times, while the Mann–Whitney score rank test was used for all the cross-comparison tests, that is, between individuals of different treatment groups over the same period of time. We used the Wilcoxon exact test to analyze weight, age, and BMI.
Results
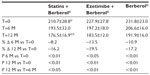
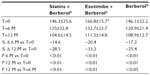
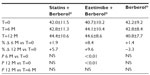
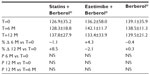
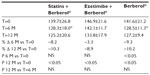
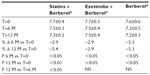
A total of 45 patients (their features are shown in Table 1) diagnosed with type 2 diabetes and hypercholesterolemia and affected by statin intolerance, were enrolled in the trial. All enrolled individuals completed the study. As shown in Tables 2–5, the patients’ lipid profile, characterized by suboptimal control on enrollment, improved in all groups in terms of TC and LDL-C, improved only in patients treated with ezetimibe and Berberol® in terms of HDL-C, and remained unchanged in all the groups in terms of TG. More specifically, as described in Table 2, after 6 and 12 months of treatment, Berberol® reduced TC by about 6% and 16%, respectively, in the statin group. When Berberol® was added to ezetimibe, TC reduction amounted to about 13% and 20% after 6 and 12 months, respectively. In the control group, in which only Berberol® was administered, TC reduction amounted to about 11% and 17% after 6 and 12 months, respectively. A significant difference is observable in Table 2 at T=0 between the Statins + Berberol® group and the other two groups; this same significant difference remains after 1 year of treatment versus Berberol® alone. The differences at T=0 are not due to enrollment mistakes, but to: 1) the difference in potency of the drug used (ie, the effect of statins being more evident than the one exerted by ezetimibe) and 2) the subjects of the Berberol® alone group were still untreated at T=0. The difference observed at T=12 versus Berberol® alone may reflect the initial difference or it is due to 1 year treatment with a statin. With regard to Table 3, Berberol® reduced the LDL-C value in the statin group by about 15% and 28% after 6 and 12 months of treatment, respectively (Table 3). When Berberol® was added to ezetimibe, this reduction increased to about 20% and 33% after 6 and 12 months, respectively. In the control group, in which only Berberol® was administered, the reduction amounted to about 17% and 26% after 6 and 12 months, respectively. As described above, the significant difference at T=0 between ezetimibe and the other two groups is due to the different drug potency and to the fact that at T=0, subjects enrolled to be treated with Berberol® alone were characterized by less severe hypercholesterolemia. No changes in HDL-C (Table 4) were observed in the statin and control groups, but this parameter decreased by less than 10% in the group where the tested treatment was added to ezetimibe. No statistically significant changes in TG were observed in any of the groups (Table 5). Glycemic control, which was suboptimal on enrollment, improved in all groups as shown in Tables 6 and 7. The improvement, modest in terms of fasting glucose, seemed to be more evident as far as HbA1c was concerned. More specifically, fasting glucose values reduced by about 10% in all groups after 12 months of treatment. This reduction appeared to be significant after 6 months only in the control group treated with Berberol® (Table 6). HbA1c values (Table 7) reduced significantly in all treatment groups and at all times considered, and ranged from about 3% to more than 5%. During the whole length of the study, none of the patients showed any CPK increase between three to ten times over the ULL, any elevation in transaminase values between three to five times, or the onset of asthenia, myalgia, or rhabdomyolysis. Tolerability data (Table 8) shows that two subjects of the statin group complained of cramps, one reported a few days of headache, and one a few days of constipation. Headache and asthenia were also side effects reported (one out of 15 per group) in the other two groups. All reported side effects lasted a few days and did not prompt the relevant subjects to interrupt the treatment. Compliance (Table 9) was overlapping in the two groups in which three tablets per day were prescribed (statin or ezetimibe + Berberol®) for lipidic control. Compliance was slightly better in the group treated with Berberol® alone, in which prescription included only two tablets a day.
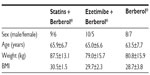  | Table 1 Features of participants on enrollment |
  | Table 8 Tolerability assessment (N=15/group) |
  | Table 9 Compliance assessment (N=15/group) |
Discussion
Statin intolerance is certainly one of the major hindrances to successful therapy with statins. Use of statins may result in optimal lipidic control in most patients suffering from hypercholesterolemia. In some cases, however, their iatrogenicity causes a lack of adherence to the therapy, or in other cases, prompts the dose to be reduced to a quantity that is unable to determine muscular sufferance or to increase CPK and/or liver transaminase. This dose reduction is obviously responsible for an unchanged lipid level, the decrease of which would reduce the cardiovascular risk by a significant degree. Due to these considerations, it is thus important to pursue the search for active ingredients other than statins so as to be able to provide effective therapy to statin-intolerant patients. Ezetimibe is a cholesterol medication that is especially useful when it is considered as an add-on treatment to the administration of low-dose statins in patients in whom reduction of the statin dosage results in the disappearance of the typical symptoms of statin intolerance. Its use in monotherapy, as an alternative to the use of statin, could be insufficient to ensure a reduction of cholesterol to such levels as to reduce the cardiovascular risk to a significant degree; hence the need for other alternatives, in particular when the patient is intolerant to statins even when the latter are administered in low doses. Berberine is certainly one of the most effective alternatives. It is an alkaloid widely used as a dietary supplement throughout Europe and North America owing to its cholesterol-lowering activity, which is linked in particular to the stabilization of the messenger carrying the code for the LDL-C receptor. This effect may result in an increase of the number of receptors per cell by a factor of 2.6.8 Another undoubtedly important mechanism for its cholesterol-lowering activity is its role played in the down-modulation of a protease known as Proprotein convertase subtilisin/kexin type 9 (PCSK9), which in turn, is involved in the destruction of LDL-C receptors when they are in the intracytoplasmic phase.20 Berberine, from a molecular point of view, acts on PCSK9 in the opposite way to statins,20 and this behavior makes it a therapeutic possibility in statin-sensitive patients. In fact, it can be added to treatment with statins in individuals who can tolerate low doses of statins and can be added to ezetimibe in statin-intolerant individuals to enhance the clinical efficacy of the latter. According to a paper published by a number of authors,21 who reported that berberine, used in monotherapy, was able to reduce LDL-C by over 20% in those cases where cholesterolemia was not too serious, berberine may as well be assumed as a single therapy. The results of our work seem to head toward this direction. The administration of berberine, formulated with silymarin to reduce its intestinal P-gp–mediated extrusion,14 in both patients being treated with low-dose statin or ezetimibe and patients not undergoing any cholesterol-lowering therapy, produces a significant effect on TC. After 1 year, the individuals of the three groups, starting from a baseline TC value that was certainly badly controlled (between 211 and 232 mg/dL on average), were found to have definitely better controlled TC levels, with values ranging 177–192 mg/dL (mean value). The results concerning the LDL-C values were still more important. In the three groups, starting from mean baseline LDL-C values of 146–176 mg/dL, the test product led to values of 105–110 mg/dL approximately, with reductions amounting to about 30% when used as an add-on therapy. The improvement of the HDL-C values was not so significant. This parameter was found to have improved only in the ezetimibe group. The lack of any improvement in the other two groups may be interpreted as a confirmation of berberine inefficacy as had already been reported by our team and some other authors,8,15,18,21 but not all.16,17 The HDL-C reduction observed in our trial in the ezetimibe group is likely to be essentially due to ezetimibe alone. In our opinion, however, the lack of any efficacy on the TG parameter is simply astonishing. TG parameter did not appear to be altered at enrollment, however literature often reports the lowering effect of berberine on TG even when the parameter seems to be “normal”.8,15,18,21 Our hypothesis is that berberine is much more efficient on the TG parameter as the latter appears to be altered. The difficult handling of the lipidic situation in statin-sensitive patients is certainly also a problem in the case of type 2 diabetes. Individuals with diabetes often exhibit an altered lipidic state, and a good percentage of them are statin-sensitive to varying degrees. From this point of view, resorting to berberine is also beneficial owing to its antihyperglycemic properties, as widely described in literature.6,7,15,18,21 This is also evident from the results of our work, in which administration of Berberol® produced significant improvements of the glycemic profile in all treatment groups; in fact, it improved baseline glycemia by 10% and HbA1c by more than 5%. These values are of particular importance when one considers that all the patients were already undergoing ‘multi-drug’ therapy aimed at controlling diabetes. The present study undoubtedly has some limitations. The lack of a direct comparison between placebo and Berberol® in randomized, statin-intolerant subjects, the lack of a group treated with low-dose statin or ezetimibe alone, the lack of a double-blind design, and the small number of participants made it impossible to arrive at definite considerations. In spite of this, its results clearly point to significantly lipid-lowering, antidiabetic pharmacological activity, which has been thoroughly described in dozens of other trials; from this point of view, our paper somehow confirms their results once again. Finally, if we consider the good tolerability profile of berberine, especially if formulated in such a manner as to bypass the problem arising from the presence of P-gp in the enterocytes,13,22 it may be considered a valid opportunity to obtain the best lipidic control in statin-intolerant patients, in particular when they are also affected by diabetes.
Conclusion
On the basis of this preliminary study, it may be confirmed that use of a fixed combination of silymarin from S. marianum added to berberine from B. aristata and administered as an add-on therapy in the form of a nutritional supplement in tablets (Berberol®), is effective in improving lipidic and glycemic profiles in statin-intolerant patients affected by diabetes.
Acknowledgment
The authors wish to thank Dr Paolo Risso for the statistical analysis of the results.
Disclosure
FDP is the main formulator and patent sole inventor of Berberol®. The other authors report no conflicts of interest in this work.
References
Sharma SB, Garg S. Small dense LDL: risk factor for coronary artery disease (CAD) and its therapeutic modulation. Indian J Biochem Biophys. 2012;49(2):77–85. | |
Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA. 2013;310(22):2451–2452. | |
Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis. 2013;23(9):871–875. | |
Alla VM, Agrawal V, DeNazareth A, Mohiuddin S, Ravilla S, Rendell M. A reappraisal of the risks and benefits of treating to target with cholesterol lowering drugs. Drugs. 2013;73(10):1025–1054. | |
Catapano AL, Farnier M, Foody JM, et al. Combination therapy in dyslipidemia: where are we now? Atherosclerosis. 2014;237(1):319–335. | |
Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57(5):712–717. | |
Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285–292. | |
Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–1351. | |
Chen W, Miao YQ, Fan DJ, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12(2):705–711. | |
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002; 91(4):193–197. | |
Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32(4):405–412. | |
Chae HW, Kim IW, Jin HE, Kim DD, Chung SJ, Shim CK. Effect of ion-pair formation with bile salts on the in vitro cellular transport of berberine. Arch Pharm Res. 2008;31(1):103–110. | |
Shan YQ, Ren G, Wang YX, et al. Berberine analogue IMB-Y53 improves glucose-lowering efficacy by averting cellular efflux especially P-glycoprotein efflux. Metabolism. 2013;62(3):446–456. | |
Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev. 2004;36(1):57–104. | |
Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab Syndr Obes. 2012;5:213–217. | |
Derosa G, Bonaventura A, Bianchi L, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther. 2013;13(11):1495–1506. | |
Derosa G, Bonaventura A, Bianchi L, et al. Effects of Berberis aristata/Silybum marianum association on metabolic parameters and adipocytokines in overweight dyslipidemic patients. J Biol Regul Homeost Agents. 2013;27(3):717–728. | |
Di Pierro F, Putignano P, Villanova N, Montesi L, Moscatiello S, Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharmacol. 2013;5:167–174. | |
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48(3):206–208. | |
Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008; 201(2):266–273. | |
Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79(6):437–446. | |
Shan YQ, Zhu YP, Pang J, et al. Tetrandrine potentiates the hypoglycemic efficacy of berberine by inhibiting P-glycoprotein function. Biol Pharm Bull. 2013;36(10):1562–1569. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.