Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Cinnamaldehyde and cinnamaldehyde-containing micelles induce relaxation of isolated porcine coronary arteries: role of nitric oxide and calcium
Authors Raffai G, Kim B, Park S, Khang G, Lee D , Vanhoutte PM
Received 25 October 2013
Accepted for publication 22 December 2013
Published 21 May 2014 Volume 2014:9(1) Pages 2557—2566
DOI https://doi.org/10.2147/IJN.S56578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Gábor Raffai,1 Byungkuk Kim,1 Sanga Park,1 Gilson Khang,1 Dongwon Lee,1 Paul M Vanhoutte1,2
1World Class University, Department of BIN Fusion Technology, Chonbuk National University, Jeonju, Jeonbuk, South Korea; 2Department of Pharmacology and Pharmacy and State Key Laboratory for Pharmaceutical Biotechnology, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, Special Administrative Region, China
Background and purpose: Cinnamaldehyde, a major component of cinnamon, induces the generation of reactive oxygen species and exerts vasodilator and anticancer effects, but its short half-life limits its clinical use. The present experiments were designed to compare the acute relaxing properties of cinnamaldehyde with those of self-assembling polymer micelles either loaded with cinnamaldehyde or consisting of a polymeric prodrug [poly(cinnamaldehyde)] that incorporates the compound in its backbone.
Methods: Rings of porcine coronary arteries were contracted with the thromboxane A2 receptor agonist U46619 or 40 mM KCl, and changes in isometric tension were recorded.
Results: Cinnamaldehyde induced concentration-dependent but endothelium-independent, nitric oxide synthase (NOS)-independent, cyclooxygenase-independent, soluble guanylyl cyclase (sGC)-independent, calcium-activated potassium-independent, and TRPA1 channel-independent relaxations. Cinnamaldehyde also inhibited the contractions induced by 40 mM KCl Ca2+ reintroduction in 40 mM KCl Ca2+-free solution or by the Ca2+ channel opener Bay K8644. Cinnamaldehyde-loaded control micelles induced complete, partly endothelium-dependent relaxations sensitive to catalase and inhibitors of NOS or sGC, but not cyclooxygenase or TRPA1, channels. Cinnamaldehyde-loaded micelles also inhibited contractions induced by 40 mM KCl Ca2+ reintroduction or Bay K8644. Poly(cinnamaldehyde) micelles induced only partial, endothelium-dependent relaxations that were reduced by inhibitors of NOS or sGC and by catalase and the antioxidant tiron, but not by indomethacin or TRPA1 channel blockers.
Conclusion: The present findings demonstrate that cinnamaldehyde-loaded and poly(cinnamaldehyde) micelles possess vasodilator properties, but that the mechanism underlying the relaxation that they cause differs from that of cinnamaldehyde, and thus could be used both to relieve coronary vasospasm and for therapeutic drug delivery.
Keywords: calcium sensitivity, cinnamaldehyde, L-type Ca2+ channel, NO synthase, micelle-forming polymeric prodrug, porcine coronary artery
Introduction
Cinnamaldehyde (Figure 1A) is the main component of Cinnamomum zeylanicum or Cinnamomum cassia extracts.1,2 It possesses antithrombotic properties in vitro and in vivo3 and has anti-inflammatory4,5 and anticancer6 effects. In the kidney, cinnamaldehyde decreases the level of nonenzymatic antioxidants and increases the activity of antioxidant enzymes.7 The compound also possesses antidiabetic properties in the rat8,9 and reduces visceral fat deposition in mice fed a high-fat and high-sucrose diet.10
With regard to acute cardiovascular effects, cinnamaldehyde reduces peripheral resistance and lowers arterial blood pressure in dogs11 and rats,12 as well as increasing hind paw blood flow in mice.13 In the latter species, single doses of cinnamaldehyde cause biphasic changes in arterial blood pressure, with an initial drop followed by a pressor response.13 In vitro, cinnamaldehyde induces relaxation of rat aortae14,15 and mouse mesenteric arteries.13 Chronic (6 weeks) treatment with cinnamaldehyde protects against increases in diastolic blood pressure after induction of diabetes in Wistar rats.15,16
The oral bioavailability of cinnamaldehyde is limited, and the compound has a short biological half-life.17 Therefore, to enhance the therapeutic potential of the compound, two polymeric prodrugs were designed. One consists of polymer without incorporation of cinnamaldehyde in the backbone (control polymer)18 that self-assembles to micelles in aqueous solution (control micelle) that can be loaded with cinnamaldehyde in its core (cinnamaldehyde-loaded micelle; Figure 1B and D, top).
The second polymeric prodrug incorporates the compound in the polymer backbone [poly(cinnamaldehyde)] and also self-assembles to form micelles [poly(cinnamaldehyde) micelle; Figure 1C and D, bottom].18 The present experiments compare the vasodilator properties of cinnamaldehyde with those of those two types of micelles containing cinnamaldehyde.
Materials and methods
Tissue preparation
Porcine hearts were collected from the local slaughterhouse (Nonsan, South Korea) and placed in ice-cold Krebs-Ringer bicarbonate buffer with the following composition (in mM): 123 NaCl, 4.7 KCl, 5.5 glucose, 1.2 MgSO4, 1.6 CaCl2, 1.2 KH2PO4, 21 NaHCO3, and 0.03 Na2EDTA (control solution). The main branches of the circumflex coronary arteries were dissected free, cleaned of adherent fat and connective tissue, cut into rings (approximately 3 mm in length), and stored (less than 14 hours) at 4°C until use. In certain rings, the endothelium was removed mechanically.19
Isometric tension recording
Recording of isometric tension was performed in a multichannel organ chamber system (Panlab SLU, Barcelona, Spain). Rings of coronary arteries were transferred to organ chambers filled with 10 mL control solution bubbled with 5% CO2 and 95% O2 and maintained at 37°C. The preparations were suspended between a stationary and an adjustable stainless steel hook; the latter was connected to an isometric force transducer (Harvard Apparatus, Holliston, MA, USA). Changes in isometric force were measured, digitalized, displayed, recorded, and analyzed with an iWorx Acquisition system (model IX/408) with Labscribe2 software (iWorx Systems Inc., Dover, NH, USA) on a computer.
Initial tension was set to approximately 0.5 g and was gradually increased to 2.5 g during a 1-hour incubation period. To obtain a reference contraction at the beginning of the actual experiment, the coronary rings were exposed twice to 60 mM KCl buffer solution obtained by equimolar substitution of NaCl with KCl.
The rings were incubated with pharmacological agents for 30 minutes, and concentration-dependent responses to cinnamaldehyde or dose-dependent responses to control micelles (containing no cinnamaldehyde), cinnamaldehyde-loaded micelles, or poly(cinnamaldehyde) micelles were measured in quiescent preparation or in rings contracted with the stable thromboxane A2 (TP) receptor agonist 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619 [2 × 10−8 M] to reach approximately 50% of the reference contraction obtained with 60 mM KCl) solution or with 40 mM KCl buffer solution (equimolar substitution for NaCl).
In a subset of experiments, contractions to increasing concentrations of Ca2+ (after incubation in Ca2+-free Krebs-Ringer bicarbonate buffer containing 40 mM KCl to activate L-type calcium channels) or to increasing concentrations of the pharmacological activator of the L-type Ca2+ channels Bay K8644 were obtained in the presence of increasing concentrations/doses of cinnamaldehyde or cinnamaldehyde-loaded micelles.
Polymer synthesis and micelle preparation
Control (polymer without incorporation of cinnamaldehyde in its backbone) and poly(cinnamaldehyde) polymers were synthesized by a Michael-type addition polymerization, as described.18 The micelles were prepared daily by direct dissolution. Briefly, polymers were dissolved in methanol (50 mg/mL), and the polymer solution was added to phosphate-buffered saline (pH 7.4). After complete evaporation of methanol, control or poly(cinnamaldehyde) micelles approximately 100 nm in size18 were obtained at a concentration of 10 mg/mL. Micelles loaded with 10% cinnamaldehyde were prepared by adding cinnamaldehyde to the methanolic polymer solution.
Drugs
U46619, trans-cinnamaldehyde, indomethacin, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), 6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo[b,m][1,5,12,16]tetraazacyclotricosine-5,13-diium ditrifluoroacetate hydrate (UCL 1684), 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34), glibenclamide, catalase, 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate (Tiron), and hydrogen peroxide (H2O2) were purchased from Sigma-Aldrich Co, LLC (St Louis, MO, USA). (1R)-(+)-camphor and ruthenium red were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). 2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide (HC030031) and Bay K6844 were purchased from Tocris Bioscience (Bristol, UK). 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was purchased from Cayman Chemical Co (Ann Arbor, MI, USA). Iberiotoxin was purchased from Bachem AG (Bubendorf, Switzerland). Nifedipine was purchased from Chunghwa Chemical Synthesis and Biotech Co, Ltd (New Taipei City, Taiwan). Indomethacin was dissolved in 0.2 M Na2CO3. UCL 1684, TRAM-34, glibenclamide, HC030031, Bay K8644, ODQ, and nifedipine were dissolved in dimethylsulfoxide; when dimethylsulfoxide was used as a solvent, its concentration was less than 0.1% in the organ chambers. All other drugs were dissolved in distilled water. The concentrations of drugs are given in molar, and those of micelles are given in milligrams per milliliter in the bath solution.
Calculations and statistical analysis
Contractions are expressed as a percentage of the reference response to 60 mM KCl (100%) obtained at the beginning of the experiment. Relaxations are expressed as a percentage of the contractions to U46619 or 40 mM KCl.
To compare various treatments, areas under the curve (AUC) and half maximal effective concentration (EC50) values of the individual concentration-response curves were calculated with the log-trapezoidal method and nonlinear regression analysis, respectively, using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA).
Data are expressed as means ± standard error of the mean. One-way analysis of variance was used to define statistically significant differences between groups. Dunnett’s post hoc test was used to identify statistically significant differences (P<0.05) compared with control.
Results
In quiescent rings, cinnamaldehyde, control micelles, cinnamaldehyde-loaded micelles, poly(cinnamaldehyde) micelles, and H2O2 in the concentration/dose ranges used (see following) did not cause significant changes in basal tension (data not shown).
Cinnamaldehyde
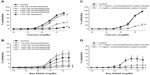
Cinnamaldehyde caused concentration-dependent (and complete) relaxations of coronary rings contracted by the TP receptor agonist U46619. These relaxations were not inhibited significantly by the removal of the endothelium (Figure 2A, top) or by L-NAME (10−4 M; inhibitor of nitric oxide synthases; Figure 2A, middle) and indomethacin (10−5 M; inhibitor of cyclooxygenases), given alone or in combination with L-NAME (Table 1). Likewise, the inhibitor of soluble guanylyl cyclase ODQ (10−5 M) had no significant effect on cinnamaldehyde-induced relaxations (Figure 2A, bottom).
Relaxations to cinnamaldehyde were not influenced significantly by the nonenzymatic antioxidant tiron (10−3 M) or by catalase (1,000 U/mL) (Figure 3A).
The TRPA1 channel inhibitors HC030031 (10−5 M or 10−4 M),20 ruthenium red (10−4 M),21 or camphor (10−4 M)22 did not significantly affect the response to cinnamaldehyde (Table 1).
Inhibitors of small- and intermediate-conductance Ca2+-activated potassium channels (UCL 1684 plus TRAM 34, respectively; 10−5 M each, given in combination), of large-conductance Ca2+ activated potassium channels (iberiotoxin; 10−7 M), or of adenosine triphosphate (ATP)-dependent potassium channel (glibenclamide; 10−6 M) did not significantly inhibit cinnamaldehyde-induced relaxations (Table 1).
Cinnamaldehyde also caused a concentration-dependent inhibition of the contractions of coronary arteries without endothelium, caused by 40 mM KCl (Figure 4A). The potency of cinnamaldehyde was significantly less in 40 mM KCl than in U46619-contracted preparations, as reflected by the comparison of the calculated EC50 values from the concentration–relaxation curves (log[EC50], 40 mM KCl: −3.22±0.06 [Figure 4A] vs U46619: −3.76±0.06 [Figure 2A, top, control]; P<0.05). Nifedipine (10−7 M) and cinnamaldehyde (32–320 × 10−9 M) inhibited the contractions induced by Ca2+ reintroduction after incubation in 40 mM KCl Ca2+-free solution (Figure 5A) or in response to increasing concentrations of the L-type Ca2+ channel opener Bay K8644 (Figure 5B).
Cinnamaldehyde-loaded micelles
Cinnamaldehyde-loaded micelles, but not control micelles prepared from the polymer without incorporation of cinnamaldehyde (data not shown), caused dose-dependent and complete relaxations of the coronary rings; the initial phase of the relaxation (caused by 10−3 to 10−1 mg/mL) was prevented by endothelium removal (Figure 2B, top). L-NAME (Figure 2B, middle), but not indomethacin (Table 1), significantly inhibited the relaxation induced by cinnamaldehyde-loaded micelles. ODQ inhibited the initial phase of this relaxation (Figure 2B, bottom), which was also inhibited by catalase, but not by tiron (Figure 3B) or HC030031 (Table 1).
Cinnamaldehyde-loaded micelles caused a dose-dependent inhibition of the contractions caused by 40 mM KCl in coronary arteries without endothelium (Figure 4B). Although the lowest dose (0.1 mg/mL) of cinnamaldehyde-loaded micelles tested was without effect, a higher dose (1 mg/mL) significantly inhibited the contractions induced by Ca2+ reintroduction in 40 mM KCl Ca2+-free solution (Figure 5C); both doses inhibited the increases in tension caused by Bay K8644 (Figure 5D).
Poly(cinnamaldehyde) micelles
Poly(cinnamaldehyde) micelles also caused dose-dependent partial relaxations of the coronary rings (Figure 2C). The relaxations to poly(cinnamaldehyde) micelles were abolished by endothelium removal (Figure 2C, top), by L-NAME (Figure 2C, middle), and by ODQ (Figure 2C, bottom), but they were not influenced significantly by indomethacin (Table 1).
Relaxations to poly(cinnamaldehyde) micelles were inhibited both by catalase and tiron (Figure 3C) but were not affected significantly by either HC030031 or ruthenium red (Table 1).
Nifedipine and hydrogen peroxide
Nifedipine relaxed 40 mM KCl-contracted coronary rings more potently than those contracted with U46619 (Figure 6).
In U46619-contracted coronary artery rings, H2O2 caused a slight, nonsignificant initial increase in tension (data not shown), followed by a concentration-dependent relaxation that was abolished by catalase (Figure 7).
Discussion
The present study compared the vascular effects of cinnamaldehyde in porcine coronary arteries with the effects in two types of cinnamaldehyde-containing micelles that were developed to enhance the therapeutic potential of the compound.
Cinnamaldehyde
The vasodilator response to cinnamaldehyde itself was analyzed first. The present results confirm that the compound, given acutely in vitro, causes relaxation of isolated arteries.13–15 The results demonstrate that the relaxation of contracted porcine coronary arterial rings is endothelium-independent. The response is insensitive to inhibitors of NOS and soluble guanylyl cyclase, as also reported in the rat aorta,15 although in the latter preparation, partial reduction of the relaxation with endothelium removal or NOS inhibition has been reported.14 Likewise, a contribution of cyclooxygenase products to the relaxation induced by cinnamaldehyde can be excluded, as indomethacin (alone or in combination with L-NAME) was without effect, as seen in different aortic preparations of the rat.14,15 Furthermore, relaxations to cinnamaldehyde were also resistant to inhibitors of hyperpolarizing (small- [UCL 1684],23 intermediate- [TRAM-34],24 and large-conductance [iberiotoxin]25), Ca2+-activated, or ATP-dependent (glibenclamide)26 potassium channels.
Cinnamaldehyde may be a natural agonist of TRPA1 channels.1,27,28 The superfamily of TRP channels includes ankyrin (TRPA1), which is expressed and physiologically active in a variety of cell types.29–33 TRPA1 channels found in the vascular cells28,34 can be activated by several physical and chemical stimuli,1,28,33–36 Because of their nonselective cation permeability,29–33 they can modulate the function of both vascular smooth muscle and endothelial cells by acting as depolarizing electrogenic channels and/or as a Ca2+ entry route.29–32 In the mouse, single doses of cinnamaldehyde cause biphasic, TRPA1-dependent changes in arterial blood pressure, with an initial drop followed by a pressor response.13 Vasodilatation induced by TRPA1 activation involves the interactions of more than one cell type in the vascular wall.28,34 However, the deletion of TRPA1 channels does not completely eliminate the relaxation to cinnamaldehyde in mouse mesenteric arteries,13 which raises the possibility that TRPA1 channels are not solely responsible for the vascular responses to the compound. The present results comfort the latter interpretation, as they demonstrate that relaxations induced by cinnamaldehyde in porcine coronary arteries are not influenced by TRPA1 channel inhibitors.20–22 Thus, unlike in other vascular areas,13,37 in porcine coronary arteries, cinnamaldehyde-induced relaxations are independent of TRPA1 channel activation. They appear, rather, to involve reduction in smooth muscle Ca2+ sensitivity and/or inhibition of voltage-gated Ca2+ channels.
The first conclusion is based on the observation that cinnamaldehyde, unlike nifedipine, relaxed U46619 contracted rings more potently than preparations stimulated with 40 mM KCl. This observation is in line with the finding that Ca2+ sensitization is for the major contributor to the U46619-induced contractions of porcine coronary arteries.38 The second interpretation is supported by the observations that the compound, similar to nifedipine, concentration-dependently inhibited contractions in response to 40 mM KCl solution, as also seen in rat aortae.14,15 High KCl solution contracts porcine coronary arteries by inducing depolarization and elevating the myoplasmic Ca2+ concentration without affecting smooth muscle Ca2+ sensitivity.38 The experiments demonstrating that the compound inhibited contractions induced by either reintroduction of Ca2+ after depletion of the activator ion in 40 mM KCl solution or the voltage-dependent Ca2+ channel activator Bay K864439 further support an inhibitory effect on voltage gated Ca2+ channels.
Cinnamaldehyde-containing micelles
Cinnamaldehyde-loaded micelles also caused complete relaxation of the coronary arterial rings, whereas poly(cinnamaldehyde) micelles caused only partial relaxation (60%–80%).
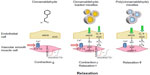
Endothelium removal and incubation with L-NAME caused only partial inhibition of the relaxations induced by cinnamaldehyde-loaded micelles, whereas relaxations to poly(cinnamaldehyde) micelles were completely dependent on the presence of endothelium and NOS activation. In both cases, the NOS activation and endothelium-dependent NO release must lead to relaxation of the vascular smooth muscle after stimulation of soluble guanylyl cyclase, as ODQ, a selective inhibitor of the enzyme,40 reduced the relaxations to cinnamaldehyde-loaded micelles and poly(cinnamaldehyde) micelles to the same extent as endothelium removal or inhibition of NOS. The present results do not explain why micelles containing cinnamaldehyde in one form or the other, but not control micelles not containing the compound, exert endothelium-dependent effects. However, we assume that because of the size of the micelles (~100 nM), they can be internalized by the endothelial cells, where they undergo intracellular acidic/lysosomal dissociation, as seen in vitro,18 leading to the release of cinnamaldehyde and micelle-forming polymer and/or its degradation product or products. The cinnamaldehyde delivery from the core of the control micelles seems to be essential for NOS stimulation and NO productions, as such an endothelium-dependent component of the relaxations was absent when cinnamaldehyde or unloaded control micelles were applied alone.
The present results also indicate that in the case of cinnamaldehyde-loaded micelles, the compound released from the micelles is responsible for the endothelium-independent component of the observed complete relaxation, as it appears to be a result of both reduced Ca2+ sensitivity and inhibition of calcium influx through voltage-dependent channels. The latter conclusion is based on the observation that cinnamaldehyde-loaded micelles inhibited the contractions induced by 40 mM KCl solution and Ca2+ reintroduction in Ca2+-free 40 mM KCl solution, as well as those by Bay K8644, in an identical manner to that of cinnamaldehyde itself.
In the case of poly(cinnamaldehyde) micelles, the relaxations were completely dependent on the presence of the endothelium and on the activity of NOS, without a smooth muscle-dependent component. The mechanism underlying the activation of endothelial NOS may be similar to that assumed for cinnamaldehyde-loaded micelles, whereby cinnamaldehyde is released by the degradation of the polymeric backbone.18 In addition to the generation of NO, poly(cinnamaldehyde) micelles (and cinnamaldehyde-loaded micelles to a lesser extent) appear to induce the production of H2O2, as tiron (scavenger of superoxide anions, the precursors of H2O2)41 and catalase (enzyme metabolizing H2O2)42 inhibited the initial phase of the endothelium-dependent relaxations. The present experiments demonstrate that, indeed, catalase abolishes the relaxant effect of H2O2 in the porcine coronary artery. The production of H2O2 is not incompatible with NOS-dependency, as this enzyme can generate H2O2 in amounts causing endothelium-dependent relaxations.43,44
Conclusion
Cinnamaldehyde, whether given directly or delivered by micelles containing the compound in their core, induces endothelium-independent relaxation of coronary vascular smooth muscle by inhibiting Ca2+ sensitivity and Ca2+ influx (Figure 8). Micelles containing the compound, either in their core or as part of their polymer backbone, cause endothelium-dependent, NOS-dependent relaxations mediated by NO (causing activation of soluble guanylyl cyclase in vascular smooth muscle) and possibly H2O2 (Figure 8). Thus, both micelles could be used for drug delivery to relieve coronary vasospasm.
Acknowledgment
This work was supported by the World Class University program (R31-20029), Ministry of Education, Science and Technology, South Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6(1):79–96. | |
Meena V, Sree Satya N, Surya Prakash DV, Sumanjali A. A review on pharmacological activities and clinical effects of cinnamon species. Res J Pharm Biol Chem Sci. 2012;3(1):653–663. | |
Huang J, Wang S, Luo X, Xie Y, Shi X. Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thromb Res. 2007;119(3):337–342. | |
Huss U, Ringbom T, Perera P, Bohlin L, Vasänge M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J Nat Prod. 2002;65(11):1517–1521. | |
Lee HS, Kim BS, Kim MK. Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem. 2002;50(26):7700–7703. | |
Kwon HK, Jeon WK, Hwang JS, et al. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009;278(2):174–182. | |
Gowder SJ, Devaraj H. Effect of the food flavour cinnamaldehyde on the antioxidant status of rat kidney. Basic Clin Pharmacol Toxicol. 2006;99(5):379–382. | |
Subash Babu P, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde – a potential antidiabetic agent. Phytomedicine. 2007;14(1):15–22. | |
Zhang W, Xu YC, Guo FJ, Meng Y, Li ML. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin Med J (Engl). 2008;121(21):2124–2128. | |
Tamura Y, Iwasaki Y, Narukawa M, Watanabe T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J Nutr Sci Vitaminol (Tokyo). 2012;58(1):9–13. | |
Harada M, Yano S. Pharmacological studies on Chinese cinnamon. II. Effects of cinnamaldehyde on the cardiovascular and digestive systems. Chem Pharm Bull (Tokyo). 1975;23(5):941–947. | |
Xu M, Yu L, Ding YY, Wang YM, Wang SW, Pei JM. [Experimental study on hypotensive effects of cinnamaldehyde in anesthetized rats]. Chinese Heart J. 2006;18(3):272–276. Chinese. | |
Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. 2010;87(4): 760–768. | |
Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006;29(12):2415–2418. | |
Xue YL, Shi HX, Murad F, Bian K. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc Health Risk Manag. 2017:273–280. | |
El-Bassossy HM, Fahmy A, Badawy D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol. 2011;49(11):3007–3012. | |
Yuan JH, Dieter MP, Bucher JR, Jameson CW. Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem Toxicol. 1992;30(12):997–1004. | |
Kim B, Lee E, Kim Y, Park S, Khang G, Lee D. Dual acid-responsive micelle-forming anticancer polymers as new anticancer therapeutics. Adv Functional Mater. 2013;23(40):5091–5097. | |
Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. | |
McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104(33):13525–13530. | |
Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25(16):4052–4061. | |
Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32(4):335–343. | |
Campos Rosa J, Galanakis D, Piergentili A, et al. Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: potent, non-peptidic blockers of the apamin-sensitive Ca(2+)-activated K(+) channel. J Med Chem. 2000;43(3):420–431. | |
Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97(14):8151–8156. | |
Galvez A, Gimenez-Gallego G, Reuben JP, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265(19):11083–11090. | |
Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987;262(33):15840–15844. | |
Bandell M, Story GM, Hwang SW, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. | |
Bodkin JV, Brain SD. Transient receptor potential ankyrin 1: emerging pharmacology and indications for cardiovascular biology. Acta Physiol (Oxf). 2011;203(1):87–98. | |
Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond). 2010;119(1):19–36. | |
Di A, Malik AB. TRP channels and the control of vascular function. Curr Opin Pharmacol. 2010;10(2):127–132. | |
Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. J Physiol. 2011;589(Pt 7):1527–1534. | |
Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118(3):337–351. | |
Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87(1):165–217. | |
Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012; 167(1):13–22. | |
Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin). 2008;2(6):429–438. | |
Ogawa H, Takahashi K, Miura S, et al. H(2)S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience. 2012;218:335–343. | |
Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104(8):987–994. | |
Nobe K, Paul RJ. Distinct pathways of Ca(2+) sensitization in porcine coronary artery: effects of Rho-related kinase and protein kinase C inhibition on force and intracellular Ca(2+). Circ Res. 2001;88(12):1283–1290. | |
Schramm M, Thomas G, Towart R, Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983;303(5917):535–527. | |
Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48(2):184–188. | |
Krishna CM, Liebmann JE, Kaufman D, et al. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch Biochem Biophys. 1992;294(1):98–106. | |
Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87(4):1595–1599. | |
Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106(12):1521–1530. | |
Matoba T, Shimokawa H, Morikawa K, et al. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol. 2003;23(7):1224–1230. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.