Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 5
Characteristics of infants with positional abnormal head shapes and their physiotherapy service at an Australian community health facility
Authors Leung A, Watter P, Gavranich J
Received 7 February 2014
Accepted for publication 13 March 2014
Published 16 July 2014 Volume 2014:5 Pages 83—92
DOI https://doi.org/10.2147/PHMT.S61989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Amy Leung,1 Pauline Watter,2 John Gavranich3
1Department of Physiotherapy, Royal Children's Hospital, 2Physiotherapy Division, School of Health Rehabilitation Science, The University of Queensland, Brisbane, Queensland, Australia; 3Child and Family Health Services, West Moreton Health Service District, Queensland, Australia
Purpose: There is limited biographic information regarding infants presenting with abnormal head shape in Australia and little discussion of the effect of different cutoff values for diagnosis of plagiocephaly. This study aimed to 1) describe the biographic characteristics of infants with positional abnormal head shapes referred for physiotherapy management; 2) explore their access to physiotherapy services and intervention outcomes; and 3) explore the impact of using different modified Cranial Vault Asymmetry Index (mCVAI) cutoff points in plagiocephaly classification.
Patients and methods: This retrospective community health record audit included the total cohort of infants referred over concerns about abnormal head shape to a pediatric physiotherapy service at a community health center in Australia from January 2004 to December 2007 (N=126 valid cases). Data retrieved included: demographic data; birth history; positioning; initial physiotherapy assessment; and factors associated with physiotherapy intervention and outcomes.
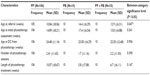
Results: Of the 126 charts (65 males), 106 infants (84.1%) presented with plagiocephaly, ten (7.9%) with brachycephaly, and ten (7.9%) with combined deformities. Most biographic data from this study were similar to those reported in the literature. The mean age ± standard deviation (SD) of infants at referral was 11.29±7.84 weeks, with about 4-weeks wait for assessment. For the plagiocephalic group, there was significant reduction in mCVAI mean value from assessment (-5.44%±2.95%) to discharge (-4.41%±2.66%) (t[df=60] =-5.396; 95% confidence interval [CI]: -1.66%, -0.76%; P<0.001) and significant change in the Argenta Clinical Classification categories (P<0.001) after physiotherapy intervention. There was a reduction of approximately 10% in infants classified with significant plagiocephaly when the mCVAI cutoff point increased by 1%.
Conclusion: Characteristics of Australian infants presenting with plagiocephaly, brachycephaly, and combined conditions were similar to other reports. Infants with positional head deformities can benefit from physiotherapy intervention. The cutoff point of mCVAI at -6% is proposed to be appropriate for the provision of ongoing physiotherapy service.
Keywords: plagiocephaly, brachycephaly, modified cranial vault asymmetry index, cutoff point
Introduction
Throughout developed countries, there has been an increased incidence of abnormal head shape reported dating to 1992, when since parents were advised to put infants to sleep on their back to reduce the risk of Sudden Infant Death Syndrome (SIDS).1–5 There was a reported change in infant sleeping position practice from about 70% in prone position in 1992 to 74% in supine position in 2009,6 and this has been associated with a reduction in SIDS deaths.7 Australian health practitioners following this “Back to Sleep” recommendation have noted a resulting dramatic increase of infants with a flat spot on the occiput and consequent increased demands on service providers. The etiology of the flat spot is commonly due to positional issues. The shape of the head is described as plagiocephaly when the flat spot is on one side of the occiput and as brachycephaly when the flat spot is in the central portion of the occiput. Therefore, the terms positional plagiocephaly (PP) and positional brachycephaly (PB) were used in this article. A combination of plagiocephaly and brachycephaly can coexist, with a characteristic wide and asymmetrical head shape.8,9 Positional molding causes the vast majority of plagiocephaly.10
Decades ago, general consensus was that plagiocephaly was only transient, that spontaneous recovery occurred once the infant became mobile, and that there was no obvious developmental implication.11,12 Only a small percentage of children exhibit residual asymmetry into their childhood.13,14 The association of PP with psychomotor developmental delay,14–17 difference in the auditory processing domain (which may indicate brain dysfunction),18,19 optometric problems,20 visual field defects,21 and temporomandibular joint asymmetry22 have been reported; however, to date, no causal relationship between PP and these unfavorable outcomes has been clearly established. Furthermore, there has been concern about the psychosocial impact on the child who has an abnormal head shape.23 This empirical evidence of developmental and multisystemic dysfunctions has caused concerns in parents and health professionals.
There has been discussion around both the tools available for measuring PP and the use of different cutoff points to classify significant and nonsignificant plagiocephaly. Hutchison et al reported that at the 4-month age, there was approximately a 10% change in PP prevalence for every 1% change in cutoff point in their study.13 McGarry et al advocated for a standard classification of the plagiocephaly categories, to aid appropriate and consistent treatment pathways and evaluation of outcomes.24 This standardized measurement tool should be accurate, reliable, and easily applicable in both tertiary and community settings. The anthropometric measurement tools using sliding calliper,25,26 thermoplastic materials27,28 and flexicurve4,29 seem to satisfy these criteria.
A comprehensive study reported that the incidence of PP varies with age, being present in 16% of infants at 6 weeks, 19.7% at 4 months, 9.2% at 8 months, 6.8% at 12 months, and 3.3% at 24 months.13 More recently, van Vlimmeren et al reported differing figures, suggesting that PP was found in only 6.1% of newborns and in 22.1% of 7-week-old neonates.30 Both authors indicated a high incidence, of around 20%, between 7–16 weeks. PP is considered to be a preventable problem, and implementation of preventive strategies by parents is advocated.23,31–33 These strategies are promoted by general practitioners, child health nurses, and physiotherapists.13,34–36
Conservative treatment of PP, including repositioning, physiotherapy, and orthotic therapy, is recommended.37 Saeed et al advocated that physiotherapy is particularly effective for PP associated with sternocleidomastoid muscle imbalance and/or tightness.38 The decision regarding orthotic therapy is mainly driven by cosmetic concerns, which are largely based on the subjective judgment of parents. This subjective judgment does not always correlate to the severity of the deformity in objective measurements.39 In addition, there are possible side effects of the orthotic device, including contact dermatitis, pressure sores, skin irritation, and potential social and psychological stigma.38 In Australia, the cost of orthotic therapy ranges from A$550 to A$600.
The demographic information for infants presenting with abnormal head shapes is not well described in the Australian population. Further, there is only limited reporting of initial physiotherapy assessment, intervention, and outcomes of these infants, as well as of the impact of cutoff value in establishing the diagnosis.
The aims of this study therefore were 1) to describe and compare the characteristics of infants with PP, PB, and combined plagiocephaly and brachycephaly (PP + PB) referred for physiotherapy management in an Australian community health center; 2) to explore their access to physiotherapy services and intervention outcomes; and 3) to explore the impact of using different modified Cranial Vault Asymmetry Index (mCVAI) cutoff points in PP classification and physiotherapy service.
Methods
Study design
This was a retrospective community health record audit of the total cohort of infants who were referred due to concerns about abnormal head shape to a pediatric physiotherapy service at a community health center in Australia from January 2004 to December 2007. The principal researcher was the sole pediatric physiotherapist treating these infants and was an experienced pediatric clinician. It can be considered that the physiotherapy assessment and treatment of these infants were consistent across the study period. Infants were excluded if 1) there was no initial physiotherapy assessment; 2) they were diagnosed with syndromes or other musculoskeletal/neurological disorders; or 3) if the parent declined head shape assessment. Ethical approval was granted from the Medical Research Ethics Committee at the local Health Service District Human Research Ethics Committee and The University of Queensland, in accordance with the National Health and Medical Research Council guidelines.
Measures
Data was retrieved from the medical records including: demographic data (date of birth, suburb of residence, sex, singleton/twin, birth order); birth history (place of birth, birth weight, gestation age, delivery type, perinatal factors, stay in special care nursery); positioning data (sleeping position, time and frequency of tummy play, alternating head position during sleep); initial physiotherapy assessment record (PP measurements, including mCVAI,29 modified Cranial Index,40 and Argenta Clinical Classification [ACC];41 musculoskeletal measurements of active and passive neck range of movement; developmental stages; and neurological findings, including muscle tone, ankle clonus and Babinski sign); factors associated with physiotherapy intervention and outcomes (age at initial referral, age at physiotherapy assessment, reason for not attending physiotherapy assessment, number of physiotherapy sessions, modality of physiotherapy, compliance with physiotherapy program, change in PP measurements, and reason for discharge).
The mCVAI, cranial index,40 and ACC41 were used at the time of the study period to identify significant abnormal head shape in the Community Health Paediatric Physiotherapy Service. Loveday and de Chalain4 introduced the Cranial Vault Asymmetry Index (CVAI) as a clinical tool to document changes in cranial asymmetry, in an intervention study. In this procedure, a flexicurve was used to obtain a circumferential head tracing, and two diagonal lines drawn30 from the anteroposterior pole (central line). The CVAI was calculated as the difference in the length of the diagonals divided by the shorter diagonal, multiplied by 100%. The cutoff point for significant plagiocephaly was set at −3.5%, although no clear rationale is evident. The principal researcher later made a minor modification in the reference points in the measurement in order to improve accuracy, consistency, and clinical efficiency. Therefore, the mCVAI was reported as used in this study, with procedure and psychometric properties reported elsewhere.29 The cranial index is the measurement of cranial proportion, which is the maximum cranial width divided by the maximum cranial length multiplied by 100%.40 This original formula continues to be used in modern assessment, although some changes were suggested in how cutoffs are interpreted. The head shape is described as dolichocephalic when the cranial index is <73.49%, as mesocephalic when the cranial index is 73.5%–80.49%, and as brachycephalic when the cranial index is >80.5%.42 Hutchinson et al showed that infants who were supine sleepers had wider heads and suggested the cutoff point to be 93%.43 The 93% cutoff point was used in this study. A qualitative clinical description of plagiocephaly and brachycephaly was reported by Argenta et al, suggesting that five grades of plagiocephaly can be identified according to clinical presentations and severity.41 From minimal to severe, grade 1 has occipital flatness, grade 2 adds ear asymmetry, grade 3 adds forehead asymmetry, grade 4 adds facial asymmetry, and grade 5 adds abnormal cranial vertical growth. For PB, Argenta et al describes three grades: grade 1 has central occipital flatness, grade 2 adds widening of the posterior skull, and grade 3 adds vertical head growth or temporal widening.41 The ACC serves as a qualitative measurement for abnormal head shape and is supported in the literature by moderate interrater and intrarater reliability.44
Intervention
The physiotherapy intervention aimed to alleviate musculoskeletal limitations, to avoid prolonged time when pressure is exerted on the flat spot, and to promote gross motor development. The treatment modalities included stretching exercises if there was sternocleidomastoid muscle tightness or limited passive neck rotation range of movement; facilitation of active neck rotation to the nonpreferred side; advice on repositioning strategies so that the infant was not resting the head on the flat spot for prolonged periods of time; and demonstration of various play positions to promote gross motor development. Advice and activities were individualized, therefore the parents received tailored programs, which could be implemented in their daily routine. Usually infants were reviewed monthly except for severe cases, where fortnightly appointments were provided. At discharge from the physiotherapy, the infant should have demonstrated age-appropriate development, full neck range of movement, and improvement in head shape.
Data analysis
The Statistical Package for Social Sciences (SPSS® Version 19; IBM, Armonk, NY, USA) was used to analyze data, evaluating the distribution and frequency of each variable as well as the types and frequency of use of physiotherapy services, and the relationships between the variables. The Kruskal–Wallis test was used to analyze nonparametric data to explore differences between groups. For parametric data, t-test and analysis of variance were used to test between-group differences. Alpha was set at P≤0.05.
Results
There were total of 156 community health records audited; thirty records were excluded according to exclusion criterion 1 (n=28); criterion 2 (n=1) and criterion 3 (n=1). Of the 126 charts (65 males), 106 infants (84.1%) presented with PP, ten (7.9%) with PB, and ten (7.9%) with PP + PB. Not all characteristics were described in every health record, and the frequency of each measure is reported as a fraction of the number for whom data was available.
Demographic and biographic information
Almost 90% of infants resided within the catchment suburbs; 58.3% were born in the local public hospital; 81% were referred by child health nurses; 93.5% had no special care nursery admission. The demographic and biographic characteristics of the total cohort of infants, according to head shape categories, are presented in Table 1. The mean birth weight ± standard deviation (SD) of the PP group was 3,327.66±621.52 g, of the PB group was 3,366.50±415.09 g, and of the PP + PB group was 3,488.75±600.91 g. The mean gestational age of the PP group was 38.83±2.03 weeks, of the PB group was 39±1.16 weeks, and of the PP + PB group was 39.33±1.00 weeks. There was no significant difference between head shape groups for birth weight and gestational age.
Following SIDS recommendations, most of the infants (88.7%) were put to sleep on their back. Only 38.9% of mothers reported that their infants tolerated tummy play position well, while 59.3% disliked it but tolerated it for a while and 1.9% were very upset and unable to stay on their tummy. The mean age for first noticing a flat spot in the PP group was 5.46±4.93 weeks, in the PB group was 10.67±7.76 weeks, and in the PP + PB group was 7.75±5.7 weeks. Despite apparent differences in these times for noticing a change, there was no significant difference between groups.
Physiotherapy intervention and outcomes
For the total cohort, the mean age of infants at referral was 11.29±7.84 weeks and at initial assessment was 15.16±7.97 weeks (range 2–56 weeks). Therefore the waiting time was approximately 4 weeks. The physiotherapy service data for head shape group is presented in Table 2. Infants with PB were referred at significantly older ages compared with those with PP. The physiotherapy assessment information is presented in Table 3. More than 90% of infants had muscle tone within normal limits and no abnormal neurological signs. For neck range of movement, one-third of infants with PP presented with limited active rotation to the side opposite to the flat occiput. The distribution of performance on the age-appropriate developmental screening items (infants’ head extension in prone position, tummy play tolerance, and gross motor mobility) was similar across the three groups. However reference to Table 3 shows clearly that considerable numbers of infants experienced difficulty with each of these items.
There was a significant difference in mCVAI mean value between the PP group (−5.44%±2.95%; 95% confidence interval [CI]: 4.86%, 6.03%) and the PP + PB group (−3.36%±1.56%; 95% CI: 2.16%, 4.56%) (χ2[df=1] =4.438; P=0.035). Infants had a milder plagiocephaly in the combined presentation. In contrast, there was no significant difference in the severity of the brachycephaly between the PB group (cranial index mean =94.45%±2.36%) and the PP + PB group (cranial index mean =94.98%±3.23%). For the PP group, there was significant reduction in mCVAI mean value from assessment (−5.44%±2.95%) to discharge (−4.41%±2.66%) (t[60]=−5.396; 95% CI: −1.66%, −0.76%; P<0.001). Using −3.5% as the mCVAI cutoff point, as per Loveday and de Chalain,4 one-quarter of infants (25.3%) showed a nonsignificant PP at initial assessment. At discharge, there were 43.3% of infants with nonsignificant PP. In addition, a significant change in PP ACC categories (P<0.001) was also noted (Table 4). More than half of the infants with grade 4 had improved to grade 3, which means their facial asymmetries had been resolved. There was a significant difference (χ2[df=] =10.09; P=0.001) in the distribution of the PP ACC categories between the PP group and PP + PB group, with the PP + PB group having a lower severity of clinical features involved. This matches with the finding that the PP + PB group had milder PP quantitative measurements.
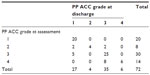  | Table 4 Change of ACC frequency at assessment and upon discharge |
All infants received repositioning strategies information, 3.2% of infants required specific developmental activities, and only one infant needed neck rotational stretching exercises. With respect to parent compliance with the home program, the majority of parents (84.5%) reported that they performed regular daily practice, 14.7% reported occasional practice, and only one parent reported rare practice. One infant was referred for orthotic therapy by the therapist, within the study period. Of the 126 infants, 29.4% of infants did not attend physiotherapy review appointments – their mean mCVAI was −6.45%±3.68% (range −1.4% to −15.7%), which was significantly (F[df=1,105] =7.405; P=0.008) worse than those infants who completed physiotherapy (−4.81%±2.42% [range 0% to −11.2%]). In a detailed examination of the data, one-third of the PP infants who did not attend follow-up appointments had mean mCVAI greater than −8%, while less than 10% of the PP group who had completed physiotherapy intervention had a similar severity.
mCVAI cutoff points
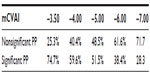
In the PP group, the effect of using different cutoff values, as reported in the literature, was explored for 99 infants with data available. The infants were allocated to either the nonsignificant PP (NSPP) group if below the cutoff point or to the significant PP (SPP) group if equal to or above, for each cutoff value reported in the literature. The distribution of infants across the categories is represented in Table 5. There was an increase of 15% of infants in the NSPP group when the cutoff was changed from −3.5% to −4.0%. Then there was a steady increase of approximately 10% of infants in the NSPP group for each 1% increase of cutoff points. Using the −3.5% cutoff point, there was no significant difference between NSPP and SPP groups in terms of number of treatment weeks and number of physiotherapy sessions. Using the −6.0% cutoff point, there was significant difference in the number of treatment weeks (F[df=1,97] =4.714; P=0.032), with the SPP group having longer treatment time than the NSPP group. For the improvement in mCVAI, there was significant difference between the NSPP and SPP group at −3.5% cutoff (F[df=1,60] =12.943; P=0.001) and at the −6.0% cutoff (F[df=1,60] =34.374; P=0.001).
  | Table 5 Distribution of PP infant group according to mCVAI cutoff points |
Discussion
Characteristics
According to the literature, among parents who were concerned enough about their infant’s head shape to seek a referral, 20% of infants presented with PB.43,45 In contrast, our study had only about 8% of infants with brachycephaly. It seems that parents in this community may have had more concern for cosmetically abnormal plagiocephalic heads, which can also involve asymmetry of facial features. We had a similar proportion of male and female infants in our study, and different ratios, of 3:2 and 2:1, are reported elsewhere.5,43,46–48 In line with other studies,30,36,43,45,49–51 our study reported right-sided plagiocephaly twice as often as left-sided in the PP group. Various explanations have been offered regarding this phenomenon, including left occipital anterior presentation at birth,52 head positional preference, or head orientation preference (HOP) to the right side during the neonatal period.13,51 In contrast, in our PP + PB group, 90% of flatness was on the left side, but this may be affected by the small number in this group. Only about 6% of our infants with PP resulted from multiple birth, lower than the 17% reported by Oh et al.48 Our infants’ mean gestational age was 38.83 weeks and birth weight was 3,328 g, close to those of 36.5 weeks and 3,460 g reported in other work.47,48
The parents’ reported mean age (5.46 weeks) of first noting the abnormal head shape was similar to that (ie, 6 weeks) of parents studied by Oh et al48 and Hutchison et al.43 However, the infants were referred for intervention at the mean age of 10.84 weeks and assessed at 14.89 weeks in our study, earlier than the 22 weeks reported in a tertiary clinic.43 This gap may be partly explained by longer waiting time in a tertiary facility compared with the primary care service in a community setting. As maximum correction of head shape depends on brain growth, which is fastest in the first year, shorter waiting periods for either referral or treatment is vital since early intervention will maximize the correctional potential.
Infants who present with persistent head turning to one side more than three-quarters of the time are described as having a HOP.46 The HOP is considered as a risk factor for PP.13,51 These infants usually have a near normal passive neck rotation range of motion but a limited active range of motion.43,46,51 Golden et al proposed that the limited active neck rotation could be due to the unilateral sternocleidomastoid muscle weakness instead of congenital muscular torticollis.50 Hutchison et al added that repetitive positioning during feeding, sleeping, and playing may also bring about a unilateral weakness in neck musculature that perpetuates the positional preference.43 Furthermore, there was a positive correlation between the degree of cervical imbalance and severity of the cranial asymmetry.48 The muscular origin of PP and PB was further investigated by Captier et al, who proposed that neurogenic hypertonia of certain neck muscles or muscle groups contributed to specific head positioning.53 Our study showed that limited active neck rotation range of motion was present in only one-third of the PP infants. This low percentage could further be explained by parents having already adopted repositioning strategies, which are routinely advised by the child health nurses in the health service district, prior to physiotherapy attendance. Although recent proposals have been that the HOP is due to a musculoskeletal issue and/or handling preference of parents, a neurosensorimotor origin has also been suggested,54–56 due to the fact that HOP was found in a majority of newborns.57–59 This head preference position is actively maintained by an underlying mechanism, not by force of gravity.60–62 Such neurosensorimotor causes of HOP in PP infants warrants further study, especially as it may impact on treatment options for infants referred with PP.
Physiotherapy intervention
In this study, infants were referred at around 2 to 3 months of age, probably due to increased awareness of the condition by both parents and primary health care professionals. The short waiting time to access physiotherapy service is another important factor for enhanced intervention outcomes since time is a crucial factor for head shape correction. The physiotherapist individualized the repositioning strategies to suit each infant’s needs and educated the parents about the natural course of the condition. We feel that this reassurance enhanced the high compliance rate of the parents to the home program. To improve head shape, infants required one to four physiotherapy sessions about a month apart, but more were required for those who had more severe PP and/or showed some delay in their development.
In this study, infants’ dislike of tummy position occurred in 58.8%–80%, with a higher rate for infants with brachycephaly. Around 10%–45% of infants showed mild delay in their development in prone position activities. Although the developmental screening of the infants in this study was a clinical examination, the findings were similar to those of Hutchison et al.43 These researchers found that 36% of PP infants showed developmental concerns in one or more domains, using the Ages and Stages Questionnaires. The most recent study by Hutchison et al showed that 23%–42% of infants showed delay mainly in the gross motor domain.63 Contrarily, Oh et al reported that 97.5% of infants with PP in their study appeared normal in their development.48 It is also suggested that supine sleepers showed later achievement in their motor milestone,64 and lack of prone play has been shown to affect developmental scores at 6 months of age.65 It is unclear whether the developmental delay is due to supine sleeping, lack of prone play, or affected by the abnormal head shape. Nevertheless, most developmental assessment tools were developed decades ago, recruiting prone sleeping infants, so their use may not be entirely appropriate to assess the supine sleeping infants of today. A review of both milestone attainment and the normative data of these assessment tools is warranted, to provide updated data, which could be affected by current infant positioning practice.
Cutoff points
A recent study attempted to generate a normative database of cranial measurements according to infant’s age and sex (N=410, age 0–12 m).9 Percentile curves were drawn according to the infant’s age and sex. More than 2,500 infants diagnosed with nonsynostotic cranial deformity were compared with the normative database. Using the CVAI, the severity of plagiocephaly was classified as follows: “mild” plagiocephaly when the CVAI fell between the 75th and 90th percentile; “moderate” plagiocephaly when the CVAI fell between the 90th and 97th percentiles, and “severe” for those above the 97th percentile. By visual analysis of the presented graphs, at around 3 months of age, the CVAI 75th percentile was approximately 4% for male infants and 3.5% for female infants. These data points support work by other authors in PP as they closely matched the cutoff points used for significant plagiocephaly in other studies.4,27
Hutchison et al proposed that the cutoff points to be 106% in their plagiocephaly measurement.13 Based on the mathematical formula, 106% is equivalent to −6% in the mCVAI. Although the measurement method is different, this cutoff point still can be used as a guide in this study, to investigate the change in outcomes related to the cutoff used. By raising the cutoff point from −3.5% to −6%, there was a reduction of nearly 50% of infants classed as having significant PP. As we demonstrated, using the −6.0% cutoff point, infants with mCVAI above −6% required more physiotherapy service, but their improvement was promising. The authors would like to propose that single physiotherapy session may be adequate for those infants with mCVAI less than −6% – on condition that there is no comorbidity, such as developmental delay, limited passive head rotational range, or head orientation preference, as well as good parent compliance with home program. Therefore physiotherapy service could be targeted to those infants with more significant PP, whose mCVAI is ≥−6%. Furthermore, primary health care professionals, such as child health nurses and general practitioners, play an important role in educating parents about repositioning strategies and in monitoring the progression of infants’ head shape. Referral to specialists, such as physiotherapists, orthotists, and/or craniofacial specialists, would then be required only for those infants who have musculoskeletal issues and/or developmental delays, who do not respond to repositioning strategies, who have worsening of head shape, or where there are queries regarding cranial synostosis.
Conclusion
The biographic characteristics of Australian infants who presented with PP, PB, and PP + PB were similar to those reported in other countries. Infants were able to access community physiotherapy service at an early age due to early referral and experienced less waiting time for community services than in tertiary facilities. Infants with positional head deformities can benefit from brief physiotherapy intervention. A cutoff point of mCVAI at −6% is proposed to be the appropriate point at which to provide ongoing physiotherapy service.
Disclosure
The authors report no conflicts of interest in this work.
References
Argenta LC, David LR, Wilson JA, Bell WO. An increase in infant cranial deformity with supine sleeping position. J Craniofac Surg. 1996;7(1):5–11. | |
Kane AA, Mitchell LE, Craven KP, Marsh JL. Observations on a recent increase in plagiocephaly without synostosis. Pediatrics. 1996; 97(6 Pt 1):877–885. | |
Turk AE, McCarthy JG, Thorne CH, Wisoff JH. The “back to sleep campaign” and deformational plagiocephaly: is there cause for concern? J Craniofac Surg. 1996;7(1):12–18. | |
Loveday BP, de Chalain TB. Active counterpositioning or orthotic device to treat positional plagiocephaly? J Craniofac Surg. 2001;12(4):308–313. | |
McKinney CM, Cunningham ML, Holt VL, Leroux B, Starr JR. Characteristics of 2733 cases diagnosed with deformational plagiocephaly and changes in risk factors over time. Cleft Palate Craniofac J. 2008;45(2):208–216. | |
National Infant Sleep Position Study (NISP). USA: Annual Survey Data 1992–2009; 2010. Available from: http://dccwww.bumc.bu.edu/ChimeNisp/Tables_in_PDF/NISP1992-2009Theusualsleepposition.pdf. Accessed April 24, 2014. | |
Task Force on Infant Sleep Position and Sudden Infant Death Syndrome. Changing concepts of sudden infant death syndrome: implications for infant sleeping environment and sleep position. Pediatrics. 2000;105(3):650–656. | |
Hutchison BL, Mitchell EA, Thomson JM. Non-synostic plagiocephaly and brachycephaly: an overview. Curr Pediatr Rev. 2006;2:33–39. | |
Wilbrand JF, Schmidtberg K, Bierther U, et al. Clinical classification of infant nonsynostotic cranial deformity. J Pediatr. 2012;161(6):1120–1125. | |
Ehret FW, Whelan MF, Ellenbogen RG, Cunningham ML, Gruss JS. Differential diagnosis of the trapezoid-shaped head. Cleft Palate Craniofac J. 2004;41(1):13–19. | |
Rekate HL. Occipital plagiocephaly: a critical review of the literature. J Neurosurg. 1998;89(1):24–30. | |
Pomatto JK, Littlefield TR, Manwaring K, Beals SP. Etiology of positional plagiocephaly in triplets and treatment using a dynamic orthotic cranioplasty device. Report of three cases. Neurosurg Focus. 1997;2(2):e2. | |
Hutchison BL, Hutchison LA, Thompson JM, Mitchell EA. Plagiocephaly and brachycephaly in the first two years of life: a prospective cohort study. Pediatrics. 2004;114(4):970–980. | |
Steinbok P, Lam D, Singh S, Mortenson PA, Singhal A. Long-term outcome of infants with positional occipital plagiocephaly. Childs Nerv Syst. 2007;23(11):1275–1283. | |
Miller RI, Clarren SK. Long-term developmental outcomes in patients with deformational plagiocephaly. Pediatrics. 2000;105(2):E26. | |
Collett B, Breiger D, King D, Cunningham M, Speltz M. Neurodevelopmental implications of “deformational” plagiocephaly. J Dev Behav Pediatr. 2005;26(5):379–389. | |
Kordestani RK, Patel S, Bard DE, Gurwitch R, Panchal J. Neurodevelopmental delays in children with deformational plagiocephaly. Plast Reconstr Surg. 2006;117(1):207–218; discussion 219–220. | |
Balan P, Kushnerenko E, Sahlin P, Huotilainen M, Näätänen R, Hukki J. Auditory ERPs reveal brain dysfunction in infants with plagiocephaly. J Craniofac Surg. 2002;13(4):520–525. | |
Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Naatanen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatr Res. 2004;56(2):291–297. | |
Gupta PC, Foster J, Crowe S, Papay FA, Luciano M, Traboulsi EI. Ophthalmologic findings in patients with nonsyndromic plagiocephaly. J Craniofac Surg. 2003;14(4):529–532. | |
Siatkowski RM, Fortney AC, Nazir SA, et al. Visual field defects in deformational posterior plagiocephaly. J AAPOS. 2005;9(3):274–278. | |
St John D, Mulliken JB, Kaban LB, Padwa BL. Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. J Oral Maxillofac Surg. 2002;60(8):873–877. | |
Roberts A. Granpa’s got a lovely head! Midirs Midwifery Digest. 2006;16:403–405. | |
McGarry A, Dixon MT, Greig RJ, Hamilton DR, Sexton S, Smart H. Head shape measurement standards and cranial orthoses in the treatment of infants with deformational plagiocephaly. Dev Med Child Neurol. 2008;50(8):568–576. | |
Mortenson PA, Steinbok P. Quantifying positional plagiocephaly: reliability and validity of anthropometric measurements. J Craniofac Surg. 2006;17(3):413–419. | |
Wilbrand JF, Wilbrand M, Pons-Kuehnemann J, et al. Value and reliability of anthropometric measurements of cranial deformity in early childhood. J Craniomaxillofac Surg. 2011;39(1):24–29. | |
van Vlimmeren LA, Takken T, van Adrichem LN, van der Graaf Y, Helders PJ, Engelbert RH. Plagiocephalometry: a non-invasive method to quantify asymmetry of the skull; a reliability study. Eur J Pediatr. 2006;165(3):149–157. | |
van Adrichem LN, van Vlimmeren LA, Cadanová D, et al. Validation of a simple method for measuring cranial deformities (plagiocephalometry). J Craniofac Surg. 2008;19(1):15–21. | |
Leung A, Watter P, Gavranich J. A clinical tool to measure plagiocephaly in infants using a flexicurve: a reliability study. Pediatric Health, Medicine and Therapeutics. 2013;4:109–115. | |
van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, L’Hoir MP, Helders PJ, Engelbert RH. Risk factors for deformational plagiocephaly at birth and at 7 weeks of age: a prospective cohort study. Pediatrics. 2007;119(2):e408–e418. | |
Neufeld S, Birkett S. What to do about flat heads: preventing and treating positional occipital flattening. Axone. 2000;22(2):29–31. | |
Otway C. Plagiocephaly and awareness, prevention and treatment. Community Pract. 2008;81(4):38–40. | |
Stellwagen L, Hubbard E, Chambers C, Jones KL. Torticollis, facial asymmetry and plagiocephaly in normal newborns. Arch Dis Child. 2008;93(10):827–831. | |
Najarian SP. Infant cranial molding deformation and sleep position: implications for primary care. J Pediatr Health Care. 1999;13(4):173–177. | |
Biggs WS. Diagnosis and management of positional head deformity. Am Family Physician. 2003;67(9):1953–1956. | |
van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, L’Hoir MP, Helders PJ, Engelbert RH. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162(8):712–718. | |
Robinson S, Proctor M. Diagnosis and management of deformational plagiocephaly. J Neurosurg Pediatr. 2009;3(4):284–295. | |
Saeed NR, Wall SA, Dhariwal DK. Management of positional plagiocephaly. Archives Disease of Childhood. 2008;93(1):82–84. | |
Feijen M, Schuckman M, Habets E, van der Hulst R. Positional plagiocephaly and brachycephaly: is there a correlation between subjective and objective assessment of cranial shape? J Craniofac Surg. 2012;23(4):998–1001. | |
Kolar JC, Salter EM. Craniofacial Anthropometry, Practical Measurement of the Head and Face for Clinical, Surgical and Research Use. Springfield, IL: Charles C Thomas; 1997. | |
Argenta L, David L, Thompson J. Clinical classification of positional plagiocephaly. J Craniofac Surg. 2004;15(3):368–372. | |
Gladwin T. The Cranial Index: A statistical study. Human Biology. 1941;13:88–102. | |
Hutchison BL, Stewart AW, Mitchell EA. Characteristics, head shape measurements and developmental delay in 287 consecutive infants attending a plagiocephaly clinic. Acta Paediatr. 2009;98(9):1494–1499. | |
Spermon J, Spermon-Marijnen R, Scholten-Peeters W. Clinical classification of deformational plagiocephaly according to Argenta: a reliability study. J Craniofac Surg. 2008;19(3):664–668. | |
Teichgraeber JF, Seymour-Dempsey K, Baumgartner JE, Xia JJ, Waller AL, Gateno J. Molding helmet therapy in the treatment of brachycephaly and plagiocephaly. J Craniofac Surg. 2004;15(1):118–123. | |
Boere-Boonekamp MM, van der Linden-Kuiper LT. Positional preference: prevalence in infants and follow-up after two years. Pediatrics. 2001;107(2):339–343. | |
Peitsch WK, Keefer CH, LaBrie RA, Mulliken JB. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110(6):e72. | |
Oh AK, Hoy EA, Rogers GF. Predictors of severity in deformational plagiocephaly. J Craniofac Surg. 2009;20 Suppl 1:685–689. | |
Mulliken JB, Vander Woude DL, Hansen M, LaBrie RA, Scott RM. Analysis of posterior plagiocephaly: deformational versus synostotic. Plast Reconstr Surg. 1999;103(2):371–380. | |
Golden KA, Beals SP, Littlefield TR, Pomatto JK. Sternocleidomastoid imbalance versus congenital muscular torticollis: their relationship to positional plagiocephaly. Cleft Palate Craniofac J. 1999;36(3):256–261. | |
Hutchison BL, Thompson JM, Mitchell EA. Determinants of nonsynostotic plagiocephaly: a case-control study. Pediatrics. 2003; 112(4):e316. | |
O’Broin ES, Allcutt D, Earley MJ. Posterior plagiocephaly: proactive conservative management. Br J Plast Surg. 1999;52(1):18–23. | |
Captier G, Dessauge D, Picot MC, et al. Classification and pathogenic models of unintentional postural cranial deformities in infants: plagiocephalies and brachycephalies. J Craniofac Surg. 2011;22(1):33–41. | |
Hopkins B, Rönnqvist L. Human handedness: developmental and evolutionary perspectives. In: Simion F, Butterworth G, editors. The Development of Sensory, Motor and Cognitive Capacities in Early Infancy: From Sensation to Cognition. East Sussex: Psychology Press Ltd; 1998:191–236. | |
Leiderman J. Mechanism underlying instability in the development of hand preference. In: Young G, Corter CM, Segalowitz SJ, Trehub SE, editors. Manual Specialization and the Developing Brain. New York, NY: Academic Press; 1983:71–92. | |
Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol Rev. 1991;98(3):299–334. | |
Gesell A. The tonic neck reflex in the human infant: Morphogenetic and clinical significance. Pediatrics. 1938;13(4):455–464. | |
Hopkins B, Lems W, Janssen B, Butterworth G. Postural and motor asymmetries in newlyborns. Hum Neurobiol. 1987;6(3):153–156. | |
Rönnqvist L, Hopkins B, van Emmerik R, de Groot L. Lateral biases in head turning and the Moro response in the human newborn: are they both vestibular in origin? Dev Psychobiol. 1998;33(4):339–349. | |
Bakker HH, Prechtl HFR. EMG activity in the neck muscles related to head and body position in the human newborn. In: Trojan S & Stastny F, editors. Ontogenesis of the brain, vol 3: the biochemical, functional and structural development of the nervous system: proceedings of the International Symposium Neuroontogeneticum tertium. Praga: Universitas Carolina Pragensis;1979:457–470. | |
Turkewitz G. Mechanisms of neonatal rightward turning bias: A reply to Liederman and Kinsbourne. Infant Behavior and Development. 1980;3:239–244. | |
Geerdink JJ, Hopkins B, Hoeksma JB. The development of head position preference in preterm infants beyond term age. Dev Psychobiol. 1994;27(3):153–168. | |
Hutchison BL, Stewart AW, de Chalain T, Mitchell EA. Serial developmental assessments in infants with deformational plagiocephaly. J Paediatr Child Health. 2012;48(3):274–278. | |
Davis BE, Moon RY, Sachs HC, Ottolini MC. Effects of sleep position on infant motor development. Pediatrics. 1998;102(5):1135–1140. | |
Monson RM, Deitz J, Kartin D. The relationship between awake positioning and motor performance among infants who slept supine. Pediatr Phys Ther. 2003;15(4):196–203. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.