Back to Journals » Clinical Ophthalmology » Volume 9
Central retinal vein occlusion resulting from anomalous retinal vascular anatomy in a 24-year-old man
Authors Kavoussi S, Kempton J, Huang J
Received 8 March 2015
Accepted for publication 7 April 2015
Published 20 May 2015 Volume 2015:9 Pages 885—887
DOI https://doi.org/10.2147/OPTH.S84214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Shaheen C Kavoussi, James E Kempton, John J Huang
Yale University Department of Ophthalmology and Visual Science, New Haven, CT, USA
Abstract: An otherwise healthy 24-year-old man presented with a painless decrease of vision in the left eye for 2 days. Best-corrected visual acuity was 20/20 in the right eye and 20/80 in the left eye. Anterior exam was unremarkable and funduscopic exam in the left eye revealed retinal hemorrhages in all four quadrants with venous dilation and tortuosity consistent with central retinal vein occlusion. Fluorescein angiography revealed delayed venous filling with neither leakage nor vasculitis. A comprehensive work-up that included infectious, inflammatory, and hypercoagulability studies was unremarkable, and magnetic resonance imaging of the orbits was unrevealing. After 2 months, best-corrected visual acuity returned to 20/20-2 in the left eye. Upon closer review of the vascular anatomy in the left eye, a bifurcation of the central retinal artery at the level of the optic disc was tightly intertwined with an undilated nasal retinal vein in a manner that appeared to compress the underlying central retinal vein, resulting in dilation and tortuosity of the remaining venous branches. The vessel wall damage, turbulent venous flow, and compressive mass effect resulting from the anomalous retinal vasculature relationship is the proposed mechanism of the central retinal vein occlusion. Careful attention to the retinal vascular anatomy is suggested to aid in assessing the risk of retinal vein occlusion in any age group.
Keywords: CRVO, young patient, negative work-up, retinal vascular anatomy
Case
A 24-year-old male non-smoker with no prior medical or ocular history presented with 2 days of painless loss of vision in the left eye. Best-corrected visual acuity (BCVA) was 20/20 in the right eye and 20/80 in the left eye. Motility, pupils, intraocular pressure, and anterior segment exam were unremarkable in both eyes. The right fundus was normal. Left fundus exam revealed retinal hemorrhages in all four quadrants with venous dilation and tortuosity and a small vitreous hemorrhage (Figure 1). There was no macular edema or thickening on optical coherence tomography. Fluorescein angiography revealed delayed venous filling without leakage, vasculitis, or choroidal ischemia; suggestive of non-ischemic central retinal vein occlusion (CRVO, Figure 2). Blood pressure monitoring and body mass index were within normal limits. Compressive, autoimmune, and hematologic work-up including complete blood count, a full lipid profile, antinuclear antibody, anti-neutrophil cytoplasmic antibody, ACE, homocysteine, syphilis studies, PROC and PROS levels, anti-cardiolipin and anti-phospholipid antibodies, Factor V Leiden, ATIII, and magnetic resonance imaging (MRI) of the brain and orbits were negative.
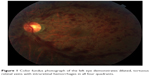  | Figure 1 Color fundus photograph of the left eye demonstrates dilated, tortuous retinal veins with intraretinal hemorrhages in all four quadrants. |
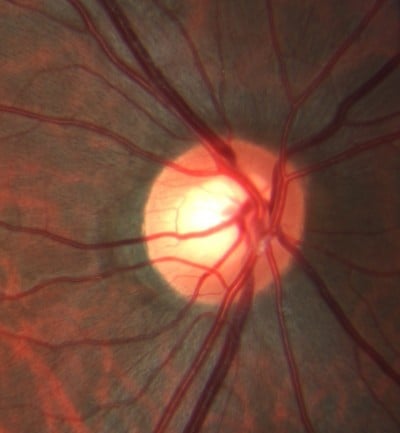
Aspirin 81 mg was started as prophylaxis, and the case did not require intravitreal anti-vascular endothelial growth factor therapy.1 At 1 week and 1 month follow-up, BCVA was 20/40 in the left eye. By 2 months, BCVA returned to 20/20-2. Residual venous tortuosity was present. Upon closer review of the nerve anatomy, the arteries were found to lie directly over the veins with the vessels tightly intertwined in the left eye (Figure 3). More specifically, an undilated nasal retinal vein was firmly wrapped around the arterial bifurcation. Since the veins that emanated from directly underneath the site of the intertwined vessels were dilated and tortuous, this anatomical relationship was postulated to be the cause of his otherwise idiopathic CRVO. No such vascular intertwining was present in the unaffected right eye (Figure 4).
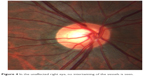  | Figure 4 In the unaffected right eye, no intertwining of the vessels is seen. |
CRVO typically occurs in patients older than 50 years with risk factors including hypertension, diabetes, and open angle glaucoma.2 CRVO in a young patient merits work-up for underlying compressive, hematologic, or autoimmune etiology.3–5 Primary pulmonary hypertension has also been reported as the hemodynamic basis of CRVO in three patients in whom significant morbidity and mortality followed the vein occlusion.6 CRVO has been reported to occur in two young patients with anomalous retinal anatomy at the level of the optic nerve, with proposed mechanism for vein occlusion to include a combination of turbulent flow, increased intravascular volume, and pressure from the arteriolar side of the circulation causing a mechanical mass effect on the venous side.7 These changes, over time, are thought to damage the vessel wall of the central retinal vein and lead to thrombosis and occlusion. These cases highlight the importance of careful inspection of the retinal vascular anatomy to aid in assessing the risk of retinal vein occlusion in any age group. Thorough work-up and co-management with the patient’s primary care physician is recommended.
Acknowledgment
This work is supported in part by an unrestricted departmental grant from Research to Prevent Blindness (RPB), Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
Pacella E, Pacella F, La Torre G, et al. Testing the effectiveness of intravitreal Ranibizumab during 12 months of follow-up in venous occlusion treatment. Clin Ter. 2012;163(6):e413–e422. | ||
No authors listed. Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1996;114(5):545–554. | ||
Gupta A, Agarwal A, Bansal RK, Agarwal A, Chugh KS. Ischaemic central retinal vein occlusion in the young. Eye (Lond). 1993;7(Pt 1):138–142. | ||
Frucht J, Yanko L, Merin S. Central retinal vein occlusions in young adults. Acta Ophthalmol (Copenh). 1984;62(5):780–786. | ||
Lahey JM, Tunç M, Kearney J, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology. 2002;109(1):126–131. | ||
Beck R, Eckard A, Ewert R, Guthoff R. Netzhauterkrankungen bei primärer pulmonaler Hypertension [Retinal diseases with primary pulmonary hypertension]. Ophthalmologe. 2003;100(9):732–735. German. | ||
Schatz H, Chang LF, Ober RR, McDonald HR, Johnson RN. Central retinal vein occlusion associated with retinal arteriovenous malformation. Ophthalmology. 1993;100(1):24–30. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.