Back to Journals » Pragmatic and Observational Research » Volume 5
Cancer pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: results from a non-interventional study
Authors Nolte T, Schutter U, Loewenstein O
Received 12 June 2013
Accepted for publication 17 September 2013
Published 19 December 2013 Volume 2014:5 Pages 1—13
DOI https://doi.org/10.2147/POR.S49793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Thomas Nolte,1 Ulf Schutter,2 Oliver Loewenstein3
1Pain and Palliative Care Centre Wiesbaden, Wiesbaden, Germany; 2Clinical Office for Pain Therapy, Marienhospital Marl, Marl, Germany; 3Regional Pain and Palliative Care Centre, Mainz, Germany
Background: Strong opioids, including oxycodone, are the most effective analgesics used to combat moderate to severe cancer pain, but opioid-induced bowel dysfunction is a relevant problem associated with the therapy. Clinical studies have demonstrated equivalent analgesic efficacy and improved bowel function in treatment with a fixed combination of prolonged-release (PR) oxycodone and PR naloxone compared to oxycodone alone in patients with nonmalignant pain. Here, we report of a prospective, non-interventional study evaluating the effectiveness and safety of PR oxycodone/PR naloxone in a subgroup of patients with severe cancer pain.
Patients and methods: Within the non-interventional multicenter study, 1,178 cancer patients with severe chronic pain received PR oxycodone/PR naloxone, dosed according to pain intensity, for 4 weeks. Recorded variables included pain intensity, patient-reported bowel function (Bowel Function Index), and pain-related functional impairment as a measure of quality of life (QoL).
Results: During treatment with PR oxycodone/PR naloxone, clinically relevant improvements in pain intensity were observed in opioid-naïve patients and in patients pretreated with weak or strong opioids, as reflected by reductions in pain scores of 51%, 53%, and 33%, respectively. Improvement in analgesia was paralleled by a significant reduction of opioid-induced bowel dysfunction in opioid-pretreated patients. The reductions in the mean Bowel Function Index of −20.5 and −36.5 in patients pretreated with weak and strong opioids, respectively, represent clinically relevant improvements in bowel function. Pain-related functional impairment decreased consistently across all seven domains, which is equivalent to a substantial improvement in QoL.
Conclusion: This subgroup analysis of cancer patients within a large non-interventional study demonstrates that treatment with PR oxycodone/PR naloxone provides effective analgesia with minimization of bowel dysfunction and improved QoL. These data extend our knowledge of the effectiveness and tolerability of PR oxycodone/PR naloxone to the population of patients with cancer under real-life conditions.
Keywords: neoplasms, pain, oxycodone, naloxone, constipation, quality of life
Introduction
Chronic pain remains a common and much feared accompaniment to cancer. A systematic review and meta-analysis of studies performed in the past 40 years revealed that pain was present in more than 60% of cancer patients with advanced or metastatic disease, and more than one-third of affected patients rated their pain as moderate or severe.1 Cancer pain is disabling across a wide range of types and phases of the disease and it has been shown to have a significant negative impact on one’s general health perception and overall quality of life (QoL).2
Opioids are the analgesics of choice for managing moderate to severe cancer pain. According to the World Health Organization’s (WHO) guidelines for the analgesic treatment of cancer pain, “weak” opioids should be prescribed for patients with mild to moderate cancer pain (WHO step 2), and “strong” opioids, including oxycodone, for those with moderate to severe pain (WHO step 3).3 Unfortunately, opioids are associated with a number of adverse effects, with constipation among the symptoms most frequently caused by an analgesic regimen administered according to the WHO guidelines.4
The term “opioid-induced bowel dysfunction” (OIBD) has been coined to describe a constellation of side effects including constipation, straining, incomplete evacuation, bloating, abdominal cramping, and pain, which is a relevant clinical problem with a strong negative impact on QoL.5–7
Laxatives do not effectively combat opioid-induced constipation, and in a multinational survey conducted among chronic pain patients with daily opioid and laxative use, the majority reported experiencing most of the OIBD symptoms at least four times a week.5 In an attempt to ameliorate constipation, approximately one-third of the patients had modified or even discontinued their analgesic medication.5 OIBD thus represents a common and debilitating side effect of opioid analgesia to which, unfortunately, only a few patients develop tolerance.6
The primary mechanism by which opioids cause OIBD is via activation of local μ-receptors in the gastrointestinal (GI) tract, resulting in changes in GI motility, secretion, absorption, and blood flow.8 Selective blockade of μ-receptors in the GI tract by administration of a locally acting opioid receptor antagonist has thus been proposed as a suitable approach to block the unwanted GI side effects of opioids, whilst retaining centrally mediated analgesia.6 While naloxone, a competitive opioid receptor antagonist, undergoes extensive first-pass metabolism resulting in negligible systemic bioavailability,9 co-administration of opioids with immediate-release oral naloxone gave rise to symptoms of withdrawal or loss of analgesia in some patients.10–12 This was not seen when both oxycodone and naloxone were administered in a prolonged release formulation with the slow release of naloxone matching the kinetic profile of oxycodone.13 In a placebo- and active-controlled Phase III trial in patients with moderate to severe low back pain, the analgesic efficacy of a fixed 2:1 combination of prolonged release (PR) oxycodone and naloxone was comparable to that of PR oxycodone alone,14 and additional clinical studies in patients with moderate to severe non-cancer pain demonstrated that PR oxycodone/PR naloxone is superior to PR oxycodone alone in terms of bowel function with no discernible loss of analgesia.15–17
In May 2006, a fixed-dose combination of PR oxycodone and PR naloxone was approved for the management of moderate to severe pain in Germany. This product (Targin®; Mundipharma GmbH, Limburg, Germany) was available in 10 mg oxycodone/5 mg naloxone and 20 mg oxycodone/10 mg naloxone tablet formulations for twice daily administration. In late 2006, a large prospective, non-interventional, post-marketing study18 was initiated to evaluate its analgesic effectiveness, effect on bowel function, tolerability, and safety during routine clinical practice. Results on 7,836 patients whose pain was due to a range of disease conditions were reported in 2010. The present paper reports a subgroup analysis on patients whose pain was primarily due to cancer.
Patients and methods
Study design
The data presented herein represent a subgroup analysis from a prospective, non-interventional, multicenter study that was conducted in Germany between October 2006 and August 2007.18 The aim of the study was to evaluate the effectiveness and safety of the fixed-dose combination of PR oxycodone/PR naloxone (Targin®) in patients with severe chronic pain who were under the care of office-based physicians. Data were to be collected during routine practice (ie, there was no intervention concerning the treatment decision or the selection and timing of diagnostic procedures).
The planned duration of the observation period was 4 weeks, with follow-up visits during treatment scheduled after 1 week (V1) and at the end of the observation period (V3). An additional visit after 2 weeks (V2) was optional for patients requiring closer monitoring of their analgesic treatment.
Information collected at the baseline visit (V0) included demographic data, medical history, underlying pain-causing disease, as well as previous analgesic and concomitant treatment, including co-analgesics and laxatives for regular use, and analgesic rescue medication. Concomitant treatments were categorized according to the groups in the “Rote Liste” (Red List) directory of approved medicinal products in Germany. Effectiveness and tolerability of the previous analgesic treatment, as rated by the treating physician, patient-reported pain intensity, pain-related functional impairment, symptoms of bowel dysfunction, and any related complaints (for example, nausea or abdominal pain) over the previous week were also documented.
Data documented during treatment included pain intensity, pain-related functional impairment and bowel function/related complaints, as well as details of analgesic and concomitant treatment, and adverse events (AEs). Data were gathered by questionnaires or interviews.
Patients and treatment
Patients with severe chronic pain that required treatment with strong opioid analgesics could be enrolled based on the decision of the treating physician to prescribe PR oxycodone/PR naloxone.18 The pain-causing underlying disease was classified according to the International Classification of Diseases, 10th Revision (ICD 10), and only patients whose pain was due to neoplasms were included in the present subgroup analysis. Patients could be included irrespective of prior analgesic treatment.
According to the contraindications listed in the prescribing information, patients were excluded if they had previously shown hypersensitivity to any of the product’s constituents, or if they had severe respiratory depression, chronic obstructive airway disease, cor pulmonale, severe bronchial asthma, paralytic ileus, moderate to severe hepatic impairment, or any other condition in which opioid therapy is contraindicated.
Administration of PR oxycodone/PR naloxone followed the dosage recommendations of the marketing authorization in existence at the time of the study (twice daily administration; maximum daily dose of 40 mg oxycodone and 20 mg naloxone). For opioid-naïve patients, the recommended starting dose was 10 mg oxycodone/5 mg naloxone twice daily. Any adjustment of the dose, the prescription of analgesic comedication, rescue medication, or laxatives was done at the discretion of the treating physician.
Outcome measures
Pain intensity was assessed using a validated German version of the Brief Pain Inventory Short Form (BPI-SF) incorporating an eleven-point numerical rating scale (NRS), with scores ranging from 0 (no pain) to 10 (worst imaginable pain).19,20 Patients were asked to record the “worst”, “least”, and “average” pain intensity that they had experienced during the preceding 24 hours, as well as pain intensity felt “right now” (ie, at the time of the interview).
Bowel function was assessed using the validated investigator-administered Bowel Function Index (BFI) rating the patient’s subjective assessment of the ease of defecation, feeling of incomplete bowel evacuation, and the level of constipation (personal judgment of constipation by the patient) during the previous week.21,22 The questionnaire incorporates an NRS that ranges from 0 (no difficulty/not at all) to 100 (severe difficulty/very strong). The BFI is the arithmetic mean of the scores for the three items. In addition, patients were asked to rate, on a five-point scale that ranged from 0 (none) to 4 (very severe), the severity during the past 24 hours of 15 bowel function-related symptoms. These included nausea, vomiting, constipation, abdominal pain, and diarrhea.
The impact of pain on patients’ QoL was evaluated using the seven domains of pain-related functional impairment that are included in the BPI-SF.19 These domains (general activity, walking ability, normal work, mood, enjoyment of life, sleep, relations with other people) are rated on an eleven-point NRS with scores ranging from 0 (no impairment) to 10 (most severe impairment), and which are summarized by calculating the arithmetic means for these seven items.
At the final visit (V3) physicians and patients assessed overall effectiveness and tolerability on a five-point scale (1= very good; 5= very bad). Physicians were also asked to assess the tolerability of PR oxycodone/PR naloxone compared with the patients’ previous analgesic therapy, with responses ranging from 1 (much better) to 5 (much worse).
Ethical considerations
The study was registered with the German Federal Institute for Drugs and Medical Devices (BfArM; study code OXN9002) and was conducted in accordance with the German Medicines Act (Arzneimittelgesetz, AMG), chapter 67, section 6 on non-interventional studies.
Statistical analysis
The set of patients to be analyzed was defined as all patients prospectively documented who presented with severe chronic pain due to a neoplasm at the initiation visit, who did not fulfill any of the exclusion criteria, and who received at least one dose of PR oxycodone/PR naloxone during the observation period.
All analyses were descriptive and exploratory. Summary measures are reported as proportions or as mean values ± standard deviation. Selected analyses were carried out for the separate groups, as defined by prior analgesic treatment: none or non-opioid-analgesics only (opioid-naïve); weak opioids; or strong opioids. Two-sided P-values for comparisons of quantitative variables between visits and between groups were calculated by paired or unpaired t-tests, respectively. McNemar’s test and chi-square tests were used to evaluate changes in binary qualitative variables between visits and between groups, respectively. Corrections for multiple testing were not made, as all results were used as purely exploratory measures.
Data were not available for all patients for all parameters at all time points. No data imputation techniques were used for missing data. Baseline data are presented for all patients who received at least one dose of PR oxycodone/PR naloxone during the observation period. For parameters assessed with the BPI-SF and the BFI, analyses were carried out both for all available data and for the cohort of patients with complete documentation of this parameter at the initiation visit and at follow-up visits V1 and V3.
Results
Patients
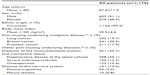
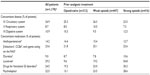
Of the 7,836 patients in the original study cohort,18 neoplasms were documented as an underlying pain-causing disease in 1,178. Patient demographics and baseline characteristics for this cohort are shown in Table 1; concomitant diseases and medications are listed in Table 2.
Previous analgesic treatment had been prescribed in 92.9% of patients, with the majority having received opioid analgesics (37.9% weak opioids, 35.3% strong opioids). The most frequently used strong opioids were oxycodone (13.4%) and fentanyl (11.3%), while tramadol was the most frequently used weak opioid (29.9%). Approximately a quarter of patients were opioid-naïve. Among these, 7.1% had not received any previous treatment, 0.9% had received co-analgesics alone, and 18.5% had received prior non-opioid analgesics (mostly dipyrone/metamizole, diclofenac, or ibuprofen). Information on analgesic pretreatment was not available for three patients.
Physician-rated effectiveness of previous analgesic therapy was “good” or “very good” in only 15.5% of patients, with lower ratings for opioid-naïve and weak-opioid pretreated patients (6.6% and 3.4%, respectively) compared to patients pretreated with strong opioids (33.9%). A “good” or “very good” tolerability rating was reported for 54% of opioid-naïve patients compared to only 32.7% and 29.4% of patients previously treated with weak or strong opioids, respectively. For previous treatment with oxycodone, analgesic effectiveness and tolerability were rated as “good” or “very good” for 56.7% and 40.3% of patients, respectively.
Treatment
Treatment with PR oxycodone/PR naloxone was initiated at 20 mg/10 mg daily in 70.9% of the patients. Higher daily doses were prescribed in 23.7% and lower doses in 5.3% of patients. At the end of the observation period (V3), 52.6% of patients were receiving 20 mg/10 mg daily and 43.5% were taking higher daily doses, mainly 20 mg/10 mg twice daily (34.6% of all patients).
Prescriptions for rescue medications (mostly morphine or metamizol) decreased between the initiation visit and the final follow-up visit for both the opioid-naïve and opioid-pretreated patients (decreases from 9.7% to 7.6% and 16.4% to 13.1% of patients, respectively). Previous prophylactic prescriptions of laxatives were reported for up to 54.8% of patients per pretreatment group (Table 2). Physicians decided to discontinue prescriptions for laxatives among almost 50% of those previously receiving laxatives, and among those previously treated with strong opioids, the proportion of patients with continued laxative prescriptions during the observation study was 23.3%.
The mean observation time was 32.9±11.4 days. A total of 138 patients (11.7%) discontinued treatment with PR oxycodone/PR naloxone, including 39 patients exhibiting insufficient effectiveness and 35 patients discontinuing due to AEs.
Pain intensity and analgesic effectiveness
Average pain intensity during the 24 hours prior to the interview at the initiation visit was 5.5±1.8 for the entire cohort. Opioid-naïve patients (5.7±1.9) and those previously treated with weak opioids (5.6±1.6) experienced more intense pain compared with those who had previously been treated with strong opioids (5.1±2.0; P<0.05 for both comparisons). A similar pattern was seen for the worst and least pain intensity during the last 24 hours, as well as for pain “right now” (ie, at the time of the interview) (data not shown). The average, worst, and least pain intensities, as well as pain intensity “right now” at V0 were lowest in the patients who had been previously treated with oxycodone (4.7±2.0; 6.0±2.1; 3.4±2.1; and 4.5±2.2, respectively).
During treatment with PR oxycodone/PR naloxone, the average pain intensity decreased consistently across all patient groups (Figure 1). The reduction in pain intensity from V0 to V3 was less pronounced in patients who had previously been treated with strong opioids (−1.8±2.3) than in the weak-opioid and opioid-naive groups, both of which experienced substantial reductions in average pain intensity (−3.0±2.0 and −3.1±1.8, respectively). The smallest decrease occurred in the patients who switched from oxycodone to PR oxycodone/PR naloxone (−1.0±2.0). Consistent reductions in worst and least pain during the 24 hours prior to the interview, as well as for pain “right now” were also seen in all analgesic pretreatment groups (data not shown). These results were paralleled by an increase from 10.4% at V0 to 35.5% at V3 in the percentage of patients from the entire cohort who said they had been pain-free during the 24 hours prior to the interview.
Prescriptions for rescue medications decreased from 14.6% of patients at V0 to 11.7% at V3. Prescriptions for rescue medications were higher for opioid-pretreated patients than for opioid-naïve patients throughout the study (13.1% versus 7.6%, respectively, at V3).
Bowel function and other complaints
At V0, constipation within the previous 24 hours was reported by 68.3% of all patients. “Mild”, “moderate”, and “severe” constipation were represented with similar frequencies (22.8%, 19.7%, and 21.6% of patients, respectively). Constipation was substantially more prevalent in patients who had received prior opioid therapy (weak opioids: 68.6%; strong opioids: 84.5%) than in opioid-naïve patients (45.7%; P<0.0001 for both comparisons). The proportion of patients who were taking laxatives or medications to combat functional GI disorders was also higher at baseline in the opioid-pretreated groups than in the opioid-naïve group (Table 2). Among patients with prior oxycodone treatment, 84.5% reported being constipated in the previous 24 hours. Of this group, 54.4% reported having used laxatives and 21.5% had used drugs for functional GI disorders.
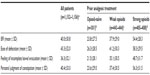
The documented BFI data support these findings. At baseline, the mean BFI was significantly higher for patients pretreated with opioids (weak opioids: 37.9±29.0; strong opioids: 54.4±28.3) than for opioid-naïve patients (23.8±27.5; P<0.0001). Mean overall and individual component BFI scores at V0 are shown in Table 3, with the data divided by the analgesic pretreatment group.
Among patients with a history of prior weak or strong opioid treatment, treatment with PR oxycodone/PR naloxone was associated with a consistent reduction in both the frequency and severity of constipation (P<0.0001; Figure 2). At V3 only 38.2% reported experiencing this symptom, representing an absolute reduction of 30% from V0. The majority of the cases of constipation that were reported at V3 were “mild” in severity (V0 versus V3: mild, 22.8% versus 30.3%; severe, 21.6% versus 1.9%). As expected, this effect was most pronounced in patients pretreated with strong opioids (Figure 2).
Overall, the mean BFI scores decreased from 39.8±30.5 at V0 to 16.2±18.5 after 4 weeks on PR oxycodone/PR naloxone (V3, P<0.0001). These data mask clear differences among the pretreatment subgroups. In opioid-naïve patients, the mean BFI fell in the normal range for nonconstipated patients in chronic pain23 throughout the observation period (data not shown). Patients on previous weak or strong opioids showed significant improvements in bowel function after both 1 week and 4 weeks of PR oxycodone/PR naloxone therapy (Figure 3). In patients who had previously been treated with oxycodone, the mean BFI score decreased from 52.9±27.6 at V0 to 18.6±20.9 after 4 weeks of PR oxycodone/PR naloxone treatment (P<0.0001). These improvements in BFI and constipation were accompanied by a decrease in the frequency and severity of other symptoms such as nausea, decreased appetite, and abdominal pain (Figure 4). The prevalence of diarrhea remained low throughout the study (9.6% and 7.3% of patients at V0 and V3, respectively).
The changes in individual components of the BFI (ease of defecation, feeling of incomplete bowel evacuation, and patients’ personal judgment of constipation) are shown in Table 4.
Quality of life and pain-related functional impairment
The average score for pain-related functional impairment in the overall patient population at the initiation visit (V0) was 6.1±1.9, with mean scores for the individual domains of pain interference ranging from 5.2±2.6 (walking ability) to 6.6±2.3 (enjoyment of life). In the domains of general activity, mood, sleep, and enjoyment of life, opioid-naïve patients tended to show greater impairment than those who had previously received opioids (weak or strong), and patients who were on prior treatment with weak opioids showed numerically greater impairments than those who had received strong opioids. The lowest overall mean pain-related functional impairment score was recorded among patients who had received prior treatment with oxycodone (5.2±2.0).
During treatment with PR oxycodone/PR naloxone (V3), pain-related functional impairment decreased consistently across all seven domains (Figure 5), which was equivalent to a substantial improvement in QoL. Magnitudes of improvements in QoL were consistently smallest for the patients who had received prior treatment with strong opioids, and they were the greatest for those who were opioid-naïve. However, even those patients who had previously received oxycodone therapy showed a substantial decrease in mean BPI-SF score (5.3±1.9 at V0; 3.7±1.9 at V3; P<0.0001), resulting from improvements in all seven domains of the BPI-SF.
Global assessment
The final assessment showed close agreement between patients and physicians in terms of their judgments of the effectiveness and tolerability of PR oxycodone/PR naloxone. Physicians and patients alike rated effectiveness and tolerability as “good” or “very good” in approximately 90% of cases. Ratings were comparable between all three patient subgroups.
Physicians rated the tolerability of PR oxycodone/PR naloxone as being “better” or “much better” than prior therapy in 86.2% of opioid-naïve patients, in 92.2% and 85.5% of patients who had previously received weak or strong opioids, respectively, and in 79.7% of patients who had received prior therapy with oxycodone, in particular.
Safety
A total of 668 AEs were reported by 233 patients. Three hundred and twenty-one events were judged to be unlikely, possibly, probably, or definitely related to the administration of PR oxycodone/PR naloxone. The most frequently reported adverse drug reactions (ADRs) were disorders of the GI tract and the nervous system (n=230 and n=36, respectively), and the majority of AEs were mild (52.4%) or moderate (30.9%) in severity.
Serious ADRs (SADRs) occurred in four patients. By the end of the study, two of these patients had recovered and one was recovering. This latter patient had developed weakness of the abducens nerve (“sixth nerve palsy”). The fourth patient died as a result of cancer shortly after the SADR (diplopia) occurred. The outcome of this SADR was therefore recorded as “unknown.” Overall, 42 patients died during the observation period, mainly due to progression of the patients’ underlying malignant disease.
Discussion
Opioids are currently the most effective analgesics that physicians have in their armamentarium, but the ability of these drugs to ameliorate pain is frequently accompanied by adverse effects on bowel function5,6,24,25 – effects that may induce patients to reduce or even discontinue opioid therapy.5 This subgroup analysis of data from 1,178 cancer patients enrolled in a large non-interventional study shows that by treatment with a fixed-dose combination of PR oxycodone/PR naloxone, cancer patients can benefit from effective analgesia with minimization of bowel dysfunction. The pivotal controlled trials conducted with PR oxycodone/PR naloxone enrolled only patients with pain due to non-malignant conditions,14–16 and the paucity of data regarding use of this treatment in cancer patients has been identified in recent literature.26 The data presented in this report help to fill this knowledge gap and extend our knowledge of the effectiveness, tolerability, side effect profile, and safety of PR oxycodone/PR naloxone to the population of patients with cancer under real-life conditions.
At baseline, the pattern of analgesic effectiveness and tolerability among the various pretreatment groups was as expected: compared with those who were opioid-naïve or who had received prior therapy with weak opioids, patients on prior treatment with strong opioids were substantially more likely to rate the effectiveness of previous analgesic treatment as “good” or “very good.” Oxycodone, in particular, showed good effectiveness; patients who had received this analgesic had the lowest values across all items of pain intensity, as reported at V0. As expected, the trend for greater analgesic effectiveness of strong opioids was reversed for tolerability; those on opioids were less likely than those who were opioid-naïve to rate the tolerability of their analgesic regimen as “good” or “very good.” Bowel dysfunction and constipation were also more severe and more prevalent in those on strong opioids than in those on weak opioids. Physicians were not asked to record their reasons for enrolling patients in this observational study. However, these data suggest that, for many opioid-treated patients, poor tolerability with bowel dysfunction rather than inadequate analgesia may have been the motivating factor for opioid switching, which is known as an option that can be used to optimize analgesia and to reduce opioid-induced side effects.27
The results of the present subgroup analysis demonstrate that treatment with PR oxycodone/PR naloxone can be an effective strategy in combating pain whilst simultaneously reducing, or even preventing, constipation and bowel dysfunction, increasing tolerability, and improving QoL in cancer patients. Switching from weak or strong opioid therapy to PR oxycodone/PR naloxone was associated with substantial reductions in pain intensity, constipation, bowel dysfunction, associated GI symptoms, and pain-related functional impairment. The anticipated improvement in bowel function that is afforded by the addition of naloxone to oxycodone – an improvement that has been demonstrated in numerous randomized, double-blind, controlled trials in non-cancer patients14–17 – was also observed in the present cohort of cancer patients. The results are also in line with those of a more recent double-blind study in which enrollment was restricted to patients with cancer pain.28 In this latter study involving 185 individuals, the mean BFI was significantly lower, and the total laxative intake was 20% lower in the PR oxycodone/PR naloxone group compared with the PR oxycodone group.
A change of ≥12 in BFI is deemed clinically meaningful,21 and a BFI of 28.8 was recently defined as the threshold for normal bowel function in chronic pain patients.23 The reductions in mean BFI, which were seen after 4 weeks of treatment with PR oxycodone/PR naloxone in patients pretreated with weak and strong opioids (−20.5 and −36.5, respectively), are thus clinically relevant improvements in bowel function. It should be noted that this improvement was achieved in the face of a decreased use of laxatives and thus reflects the true effects of fixed-dose PR oxycodone/PR naloxone in this setting. Treatment with PR oxycodone/PR naloxone led to a reduction in the BFI score below the threshold of 28.8 in patients pretreated with opioids, indicating that normal bowel function was already apparent after 1 week.
The second major finding from this study is that PR oxycodone/PR naloxone improved analgesia in patients with prior opioid monotherapy. This occurred both in patients who had previously received weak opioids, and in those who had received strong opioids. A reduction of approximately two points or 30%–36% on an eleven-point NRS, is considered to represent a clinically relevant change in pain intensity.29 The reductions in the NRS score of 51%, 53%, and 33% in opioid-naïve patients, patients pretreated with weak opioids, or patients pretreated with strong opioids, respectively, thus represent clinically relevant improvements in analgesia across patient groups. In this light, there was no evidence of a reversal of analgesia that had previously been demonstrated after the addition of immediate-release naloxone to opioid therapy,10–12 and these findings support one of the main conclusions that was made in the pivotal studies for this product:14–16 within the dose ranges administered, the analgesic efficacy of PR oxycodone is unaffected by co-administration of PR naloxone.
A decrease in pain intensity in patients who are opioid-naïve or who transfer from weak opioids to the strong opioid oxycodone, is to be anticipated, as these patients are effectively moving from step 1 or 2 to step 3 on the WHO pain ladder. A trend toward improvement in patients who had previously received strong opioids is less predictable. Given that previous treatment with oxycodone alone was rated as a highly effective analgesic in this patient population, the 20% decrease in pain – though not clinically relevant – is an interesting finding. A similar finding was observed in a subgroup analysis of patients with neuropathic pain who were enrolled in the same observational study as those described in the present paper,30 and this finding might be associated with the reduction in abdominal pain that occurred secondary to improved bowel function. Furthermore, the recorded increase in therapy tolerability might have presumably led to increased therapy compliance, resulting in better pain control.
The reduction in abdominal pain in patients who had previously received opioid therapy was accompanied by a reduction in nausea and an improvement in appetite. Similar reductions in these symptoms in association with PR oxycodone/PR naloxone therapy have been documented in the main study from which this subgroup was taken,18 and in previous controlled trials.15 These improvements may have occurred secondary to the improvement in bowel function or may stem directly from the opioid antagonistic effect of naloxone within the GI tract. Certainly, there is evidence that opioid-induced nausea is caused, in part, by a direct effect of the opioids acting on receptors within the GI tract.31–33
The third main finding of this study is that the use of PR oxycodone/PR naloxone was associated with a reduction in pain-related functional impairment and improved QoL in all pretreatment groups. The instrument that was used to measure QoL (BPI-SF) has been recommended as a pain measurement tool by the Expert Working Group of the European Association of Palliative Care,34 and the validated German version20 of this questionnaire was therefore appropriate for use in this patient population.
The improvement in QoL was most profound among the patients who were opioid-naïve at baseline, and smallest for those who had previously received treatment with strong opioids. This result concurs with the pattern of reduction in pain intensity during the observation (the magnitude of which was smallest for those patients previously treated with strong opioids), as well as with the well-established relationship between pain and QoL.35 Remarkably, QoL was also improved in patients pretreated with oxycodone, which implies that the addition of PR naloxone to PR oxycodone may improve QoL. The BPI-SF includes domains that are likely to be adversely affected by bowel dysfunction, including general activity, mood, enjoyment of life, and sleep. The addition of PR naloxone to PR oxycodone therapy may, therefore, improve QoL by affecting these domains.
The frequency and spectrum of AEs that occurred in this study were as expected for a population of patients with cancer who were receiving opioid therapy. In spite of the addition of PR naloxone to PR oxycodone therapy, ADRs were dominated by GI system events. It should be noted that almost half of the opioid-naïve patients in this study (45.7%) reported constipation at baseline. These data suggest that GI AEs are common in cancer patients; however, the absence of a control group makes it difficult to fully evaluate the safety results.
The AE data must also be viewed in conjunction with the QoL and final assessment data. As discussed above, replacement of the pre-existing analgesic regimen was associated with reductions in pain-related functional impairment and related QoL in patients across all pretreatment groups. Moreover, approximately 90% of physicians and patients rated both the effectiveness and tolerability of PR oxycodone/PR naloxone as “good” or “very good”, and this analgesic regimen was assessed by physicians as being “better” or “much better” than the pre-study regimen in the vast majority of patients; this included 80% of those previously treated with oxycodone.
The most common reason for discontinuation of the study drug was insufficient analgesic effectiveness. This is likely to have arisen because of the fact that when this study was conducted, the maximum permissible daily dose of oxycodone in this formulation was 40 mg. In contrast, other studies designed to determine the efficacy of oxycodone in combating cancer pain have recorded mean daily oxycodone doses of up to 150 mg, with daily doses of up to 340 mg allowed in some studies.36 It is, therefore, likely that the dose of oxycodone administered to some patients in this study was insufficient to adequately control their pain. In addition to the 10 mg/5 mg and the 20 mg/10 mg PR oxycodone/PR naloxone tablets, 40 mg/20 mg tablets have been approved since 2009 in many European countries. Thus, with twice daily administration, this product can now be used to administer daily oxycodone doses of up to 80 mg, allowing improved analgesia in patients with severe cancer pain.
A non-interventional study such as this has inherent limitations. Most notably, these include the absence of a control group, a lack of randomization and blinding, and the inability to ensure that all post-baseline data were recorded. This latter limitation was circumvented in the current subgroup analysis by primarily presenting post-baseline results only among patients for whom both V0 and V3 data were available. Limitations specific to the current study include the inability to control or record any co-administered analgesics, the absence of data on the specific impact of bowel dysfunction on QoL, and the relatively short study duration. However, these limitations are countered by the ability of an observational study to document the effects of a therapeutic intervention in a patient group and in a clinical situation that is representative of real-world conditions. Randomized, double-blind trials in which PR oxycodone/PR naloxone has proven to be better tolerated than, and as effective as, PR oxycodone monotherapy have already been performed in a range of patient populations.14–17,28 By documenting real-world experience with this treatment, this study adds valuable information to physicians’ knowledge regarding the treatment options available for treating cancer pain.
Conclusion
This subgroup analysis of a non-interventional study shows that a fixed-dose combination of PR oxycodone/PR naloxone allows patients with cancer pain to benefit from the systemic analgesic effects of oxycodone, while largely avoiding OIBD. Compared with the patients’ pre-study analgesic regimen, the use of PR oxycodone/PR naloxone was associated with superior analgesia, as well as improvements in bowel function and QoL. The results of this observational study thus support those of a recent randomized, double-blind trial which found that PR oxycodone/PR naloxone is well tolerated and provides effective analgesia in patients with cancer pain. These results align with those previously documented in patients with pain of non-malignant origin.
Acknowledgments
We are indebted to the physicians who documented the patients in this trial, and thank the IZKS Mainz for the statistical analysis, as well as the team at Physicians World Europe GmbH for their medical writing assistance. The study was designed and sponsored by Mundipharma GmbH, Limburg, Germany, who also provided funding for the statistical analysis and medical writing support.
Disclosure
T Nolte has received payments for consultancy/lectures from Mundipharma, Nycomed/Takeda, and Pfizer. U Schutter has received payments for consultancy/lectures from Mundipharma, Grünenthal, Pfizer, and Astra-Zeneca. O Loewenstein declares receiving payments for consultancy/lectures from Mundipharma, Grünenthal, CT/AWD, Eisai, Kade. There are no other conflicts of interest to disclose.
References
van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. | |
Kroenke K, Theobald D, Wu J, Loza JK, Carpenter JS, Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage. 2010;40(3):327–341. | |
World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva, Switzerland: World Health Organization; 1996. | |
Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93(3):247–257. | |
Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10(1):35–42. | |
Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(Suppl 5A):11S–18S. | |
Penning-van Beest FJ, van den Haak P, Klok RM, Prevoo YF, van der Peet DL, Herings RM. Quality of life in relation to constipation among opioid users. J Med Econ. 2010;13(1):129–135. | |
Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther. 1986;237(3):945–949. | |
Fishman J, Roffwarg H, Hellman L. Disposition of naloxone-7,8,3H in normal and narcotic-dependent men. J Pharmacol Exp Ther. 1973;187(3):575–580. | |
Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage. 2002;23(1):48–53. | |
Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84(1):105–109. | |
Sykes NP. An investigation of the ability of oral naloxone to correct opioid-related constipation in patients with advanced cancer. Palliat Med. 1996;10(2):135–144. | |
Reimer K, Hopp M, Zenz M, et al. Meeting the challenges of opioid-induced constipation in chronic pain management – a novel approach. Pharmacology. 2009;83(1):10–17. | |
Vondrackova D, Leyendecker P, Meissner W, et al. Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain. 2008;9(12):1144–1154. | |
Simpson K, Leyendecker P, Hopp M, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin. 2008;24(12):3503–3512. | |
Löwenstein O, Leyendecker P, Hopp M, et al. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Exp Opin Pharmacother. 2009;10(4):531–543. | |
Löwenstein O, Leyendecker P, Lux EA, et al. Efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of moderate/severe chronic non-malignant pain: results of a prospectively designed pooled analysis of two randomised, double-blind clinical trials. BMC Clin Pharmacol. 2010;10:12. | |
Schutter U, Grunert S, Meyer C, Schmidt T, Nolte T. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: a large observational study under conditions of daily practice. Curr Med Res Opin. 2010;26(6):1377–1387. | |
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. | |
Radbruch L, Loick G, Kiencke P, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manage. 1999;18(3):180–187. | |
Rentz AM, Yu R, Müller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12(4):371–383. | |
Rentz AM, van Hanswijck de Jonge P, Leyendecker P, Hopp M. Observational, nonintervention, multicenter study for validation of the Bowel Function Index for constipation in European countries. Curr Med Res Opin. 2011;27(1):35–44. | |
Ueberall MA, Müller-Lissner S, Buschmann-Kramm C, Bosse B. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res. 2011;39(1):41–50. | |
McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev. 2008;(2):CD006332. | |
Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. | |
Mercadante S. Emerging drugs for cancer-related pain. Support Care Cancer. 2011;19(12):1887–1893. | |
McNicol E, Horowicz-Mehler N, Fisk RA, et al; Americal Pain Society. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4(5):231–256. | |
Ahmedzai SH, Nauck F, Bar-Sela G, Bosse B, Leyendecker P, Hopp M. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med. 2012;26(1):50–60. | |
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. | |
Hermanns K, Junker U, Nolte T. Prolonged-release oxycodone/naloxone in the treatment of neuropathic pain – results from a large observational study. Expert Opin Pharmacother. 2012;13(3):299–311. | |
Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med. 2009;10(4):654–662. | |
Foss JF, Yuan CS, Roizen MF, Goldberg LI. Prevention of apomorphine- or cisplatin-induced emesis in the dog by a combination of methylnaltrexone and morphine. Cancer Chemother Pharmacol. 1998;42(4):287–291. | |
Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87(4):765–770. | |
Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–255. | |
Ferrell BR. The impact of pain on quality of life. A decade of research. Nurs Clin North Am. 1995;30(4):609–624. | |
Reid CM, Martin RM, Sterne JA, Davies AN, Hanks GW. Oxycodone for cancer-related pain: meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(8):837–843. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.