Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Brain meningioma with initial manifestation similar to cervical radiculopathy: a case report
Authors Huang Y, Hong C, Wu W, Li K, Chou L
Received 17 March 2014
Accepted for publication 22 April 2014
Published 25 June 2014 Volume 2014:10 Pages 1175—1181
DOI https://doi.org/10.2147/NDT.S64192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Yu-Hsuan Huang,1,* Chang-Zern Hong,2,* Wei-Ting Wu,1 Kun-Ta Li,3 Li-Wei Chou1,4
¹Department of Physical Medicine and Rehabilitation, China Medical University Hospital, 2Department of Physical Therapy, Hungkuang University, 3Department of Emergency Medicine, China Medical University Hospital, 4School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan
*These authors contributed equally to this work
Abstract: Meningiomas are the most common benign brain tumors, and are characterized by slow growth and a long asymptomatic period. Once the tumor becomes symptomatic, the various presentations may be related to the location and compression of adjacent structures. Meningioma is primarily treated through surgical intervention, and thus earlier diagnosis is likely to result in better prognosis. The symptoms of the meningioma may mimic other diseases, making precise diagnosis difficult, which will then delay treatment. We report a case of brain meningioma that showed initial signs and symptoms similar to cervical radiculopathy. The symptoms extended gradually, and the ultimate diagnosis of meningioma was confirmed based on brain-image studies. After brain-tumor excision, postoperation radiotherapy, and aggressive rehabilitation, the patient was able to perform better in daily activities.
Keywords: hemiplegia, menigioma, paresthesia, radiculopathy, rehabilitation
Introduction
Muscle weakness is a common and nonspecific complaint that encompasses a broad range of differential diagnosis. Progressive monolimb weakness is a very common symptom that occurs in any age range and in various situations, thus needing further differential diagnoses. To approach the lesions of the neuromuscular system, a careful evaluation of the motor cortex through the corticospinal tracts, anterior horn cells, spinal nerve roots, peripheral nerves, neuromuscular junction, and finally the muscle should be conducted. Detailed neurologic examination should be able to identify lesions of the central and peripheral nervous systems. Pure motor hemiparesis is a syndrome that is occasionally mimicked by central or peripheral pathologies,1 along with the etiology of cortical lesions, lacunar syndromes and basal ganglia lesions, brain-stem processes, radiculopathies, plexopathies, peripheral nerve injuries, disuse atrophy, and myasthenia gravis, among others. Cervical radicular symptoms tend to follow a dermatomal pattern, depending upon which cervical nerve root is compressed, thus producing upper-extremity radicular symptoms, such as pain, numbness, weakness, and paresthesia.2 Given the compression or inflammation of the nerve root, radiculopathy is commonly associated with a radiating pain experienced in the dermatome, myotome, or sclerotome.3 In a review of cervical radiculopathy, the clinical consequence of radiculopathy is arm pain or paresthesias in the dermatomal distribution of the affected nerve, and may or may not be associated with neck pain and motor weakness.4 The weakness attributed to central nervous systems includes common acute stroke syndromes, space-occupying lesions (such as tumors), and lesions of the spinal cord.
Meningioma accounted for more than a third of all primary central nervous system tumors reported in the US between 2006 and 2010, where the highest incidence rate (7.44 per 100,000) of the disease has been recorded.5 Meningiomas usually grow slowly, with a long initial asymptomatic phase, and may remain silent until the patient’s sudden death.6 Only 3%–6% of clinically detected asymptomatic meningiomas later become symptomatic.7 When symptomatic, intracranial meningiomas present a wide variety of symptoms arising from the compression of adjacent structures, direct invasion of or reactive changes in the adjacent brain tissue, and obstruction of cerebrospinal fluid pathways, cortical veins, or major venous sinuses.8 Symptoms and signs may include seizure disorders, raised intracranial pressure sign, classic early morning headaches, focal neurological deficits, such as motor and sensory disorders, ataxia, language dysfunction, cranial neuropathies, psychomotor symptoms, and behavioral disturbances.8 We report a case that presented initial signs and symptoms of numbness that started from the fingers, extending to the forearm, but the symptoms became atypical, resulting in an ultimate diagnosis of meningioma.
Case report
A 54-year-old man reported experiencing a gradual onset of numbness, which started from his left thumb and extended to his fingers, approximately 6 months before he was admitted. The numbness gradually progressed to his left forearm, and cervical traction was conducted at the local medical department, based on the assumption of cervical radiculopathy. The symptoms did not disappear. Three months later, intermittent headaches occurred following the numbness of the left hand, especially during defecation and coughing. At the same time, weakness in the upper-left proximal extremity, especially at the shoulder and elbow, were noted. As time progressed, he was no longer able to dress himself, because he was unable to lift his arm and forearm. However, flexion of the fingers was preserved: he could still grasp things and grip the handlebars while riding his motorcycle. Eventually, he found it difficult to carry on with his job and daily activities.
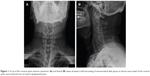
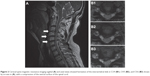
He visited our neurosurgery outpatient department 1 month prior to admission. During the initial evaluation, numbness of the left thumb, fingers, and forearm was noted. Physical examination for muscle power revealed grade IV in the left wrist joint and grade II in the left elbow and shoulder. Increasing deep-tendon reflex in bilateral lower extremities was also noted, with a positive Spurling sign at the left side and a negative Hoffmann sign. Based on an assumption of cervical radiculopathy, an X-ray of the cervical spine was performed, which showed evidence of reduced normal lordosis with a mild narrowing of the intervertebral disk spaces at almost all levels of the cervical spine, with osteoarthrosis of several apophyseal joints (Figure 1). Magnetic resonance imaging (MRI) of the cervical spine revealed a minor disk bulge at C3–C4, C4–C5, and C5–C6, with posterocentral and right paramedian compression of the ventral surface of the spinal cord (Figure 2). An electrodiagnostic test revealed normal nerve-conduction velocity in bilateral median and ulnar nerves, and normal findings in the needle-electromyography study of bilateral abductor pollicis brevis and digiti minimi muscles. After treatment using oral nonsteroidal anti-inflammatory drugs, the numbness of the upper-left limb was relieved for several days, but the weakness persisted.
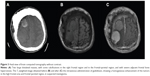
Two weeks before admission, he suffered from an unsteady gait caused by lower-limb weakness, and returned to our neurosurgery outpatient department for further evaluation. He mentioned a deformity in his skull at the right parietal bone, which he had had for years, and that he had experienced intermittent headaches while under the Valsalva maneuver within the last 3 months. Brain computed tomography was performed, revealing two large lobulated masses, with some calcifications in the right high-frontal region and another in the right frontal–parietal region and with severe adjacent frontal bone hyperostosis (Figure 3A). Brain MRI (Figure 3, B and C) with gadolinium showed two lobulated and well-defined brain tumors, one of which was in the right high-frontal area and the other in the right frontal–parietal area.
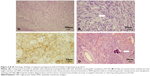
Craniectomy was performed to remove the tumors. The immunohistochemical stain and the pathology report were consistent with atypical meningioma, World Health Organization (WHO) grade II (Figure 4). After radiotherapy and 1.5 months of aggressive rehabilitation programs, muscle power in the left proximal upper limb improved from grade II to grade IV, and from grade I to grade III in the distal upper limb. The spasticity of the upper-left limb decreased from 2 to 1 in the elbow under the modified Ashworth scale. His functional status, such as his standing balance, improved from fair to good, and he could walk without the help of any device. Performance of daily activities also improved from moderate assistance to near-complete independence. The functional independence measure score improved from 71 to 106, and the Barthel index increased from 35 to 80 as the rehabilitation program ended.
Discussion
Brain-tumor symptoms can vary widely depending on the location of the mass. Possible clinical presentations may be generalized or focal signs, such as headaches, seizures, nausea, vomiting, cognitive dysfunction, extremity weakness, sensory loss, disorder of language function, and visual and hearing loss.9 A great heterogeneity in histology, recurrence rates, aggressiveness, symptoms, and survival outcomes10 exists, despite a large majority of meningioma being classified as benign lesions. Meningiomas are primarily treated through surgery, and no major changes in treatment have been achieved since the publication of the monograph by Cushing and Eisenhardt in 1938,11 except for meningiomas arising in the central skull base. Total resection for intracranial meningiomas is performed in cases of convexity, parasagittal, or falcine meningiomas.12 Therefore, early and accurate diagnosis of the symptoms and treatment of the underlying disease are important.
Based on the literature, a patient being diagnosed with intracranial meningioma with only an initial presentation of numbness in one hand and forearm is very rare. Khalatbari et al13 reported on a 56-year-old woman with brain meningioma who presented pain and paresthesia originating from the anterior upper arm and extending to her radial forearm and right thumb and index finger (corresponding to C6 radicular pain). Meningioma was finally diagnosed by brain computed tomography, which was arranged before the patient received cervical spinal surgery because of persistent headache. Our patient also developed a headache while under the Valsalva maneuver. Compared with the tumor location of our patient, the woman’s tumor growth was more central, which may have resulted in paresthesia starting from the proximal area. Vigilance should be emphasized when facing a similar case, because distinguishing between a brain tumor and radiculopathy is very difficult, and even impossible in these cases. A discussion on the distribution and pattern of the numbness and weakness may aid in arriving at a correct diagnosis in the earlier stages.
Tracing back the history of this case, our patient told of a history of cervical spondylotic radiculopathy that was not fully treated after previous cervical traction. The most common symptom of cervical radiculopathy is radiating pain in the arm due to nerve compression.14 Our patient’s symptoms appeared 6 months prior to the final diagnosis of meningioma. Initially, our patient complained about the numbness, which started from his left thumb and extended to his other fingers, and then progressed and gradually extended to his left forearm. At the same time, our patient denied any neck pain or soreness. Although cervical radiculopathy was originally suspected because of a positive Spurling sign, the distribution of the regions of numbness did not follow a dermatomal pattern, and the radiation pattern was from the distal to proximal area, which was different from the pattern in radiculopathy. Depending on the location of the patient’s meningioma (Figure 5), the numbness and paresthesia in the left hand was comparable to Penfield and Rasmussen’s first map of cortical homunculus, which compressed the primary somatosensory area in the parietal lobe of the cerebral cortex.15 However, we failed to identify this important point in the beginning. The diagnosis of cervical radiculopathy could later be ruled out based on image findings and the electrodiagnostic study.
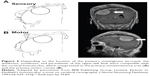  | Figure 5 Depending on the location of the patient’s meningioma (arrows), the weakness, numbness, and paresthesia of the upper-left limb were compatible with the cortical homunculus, which compressed to the primary somatosensory area (A) and the primary motor cortex (B). |
The clinical presentations of cervical radiculopathy can range from pain, numbness, and/or tingling in the upper extremity to electrical-type pains or even weakness.4 Our patient developed weakness in his upper-left proximal extremity, especially in his shoulder and elbow, but exhibited fine motor skills of his wrist and fingers, allowing him to grasp things without difficulty. At the same time, his weakness progressed severely from the proximal limbs to the distal limbs, which is unusual in radiculopathic patients. Additionally, the deep tendon reflex in the bilateral lower extremities of our patient increased, indicating an upper motor-neuron sign, and thus cervical spondylotic myelopathy (CSM) was diagnosed initially. CSM is defined clinically as a symptomatic dysfunction of the cervical spinal cord, and is the most common cause of myelopathy in adults over 55 years old, causing progressive disabilities and impairing quality of life. Cervical myelopathy must be distinguished from the less serious syndromes of cervical radiculopathy and the simple neck pains, which may also result from spondylosis.16 Some studies have concluded that a subtle gait disturbance is the most common presentation of CSM,17,18 and that a spastic gait occurs first, temporally followed by upper-extremity numbness and sensory loss, which may follow a dermatomal pattern, and often underlies complaints of loss of fine-motor control of the hands. The weakness in the proximal limb of our patient was much more severe than that in the distal area, which is a different symptom from myelopathy. In retrospect, the distribution of the weakness could be attributed to Penfield and Rasmussen’s first map of cortical homunculus, where compression of the primary motor cortex happens (Figure 5).15,19
Cervical spondylosis is the main cause of CSM. However, age-related cervical spondylosis does not always result in CSM. The radiographic incidence of cervical spondylosis in an asymptomatic population in their 70s was reported as 95% in men and 70% in women, and in patients older than 40 years old, nearly 60% had disk degeneration and 20% had foraminal stenosis.20 MRI has become the most important method in diagnosing a significant pathology in the cervical spine. However, an MRI may produce a substantial amount of false-positive findings, because asymptomatic radiological abnormalities are common in radiological imaging of the cervical spine.21 MRI findings are only part of the investigation, and one should always consider a thorough neurological examination.22 Jensen et al23 reported that 36% of 98 asymptomatic subjects had normal disks at all levels. The results of the two readings were averaged, and showed that 52% of the subjects had a bulge on at least one level, 27% had a protrusion, and 1% had an extrusion. The weak correlation between the MRI and clinical findings, such as false-positive and false-negative findings, could be influenced by both the radiologist’s and the clinician’s experience.24 In fact, the morphological findings are difficult to compare with functional results.
The WHO morphologically classifies meningiomas into three categories using a grading scheme.25 Patients harboring either grade II or III meningiomas have higher recurrence rates, varying between 29%–52% and 50%–94%, respectively. After gross total resection without postoperative radiation, grade II meningiomas are shown to have a high recurrence rate, with most recurrences occurring within 5 years after the resection.26 A retrospective case series supports the observation that postoperative radiotherapy may result in lower rates of recurrence of atypical meningiomas that have undergone initial gross total resection: actuarial recurrence rates within 5 years were close to 42% without radiotherapy and 20% with radiotherapy.27 In the current study, age and radiotherapy dosage were associated with longer 5- and 10-year actuarial overall survival rates, while size and grading of the tumor influenced the 5- and 10-year disease-free survival rates.28 In the present case, after the patient was diagnosed with meningioma, he received surgical intervention and participated in radiotherapy for the residual tumor. After aggressive rehabilitation, the muscle power in his upper-left limb increased, and the patient exhibited improved walking abilities, with near-complete independence of performing daily activities. Follow-up conducted 1.5 years later showed that the patient had no evidence of recurrence.
Conclusion
In this case report, we demonstrated a patient with brain meningioma with initial clinical symptoms similar to cervical radiculopathy. With the progression of the clinical symptoms, the diagnosis of brain meningioma was finally confirmed, and the patient was treated accordingly. Upon review of the progression of the clinical symptoms, the possibility of a brain lesion could have been identified earlier if the neurological examination was executed carefully. This examination encompasses the patient’s mental status, cranial nerve examination, motor system (muscle strength, upper or lower motor-neuron sign, and muscle tone), deep-tendon reflexes, sensation system, abnormal gaits, and coordination, and also the pattern and distribution of neurologic defects.
Acknowledgement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004).
Disclosure
The authors report no conflicts of interest in this work.
References
Lu YT, Lan MY, Liu JS, Chen WH. An unusual presentation of hypokalemic paralysis with evolving pure motor hemiparesis. J Clin Neurosci. 2011;18(5):716–719. | ||
Waldrop MA. Diagnosis and treatment of cervical radiculopathy using a clinical prediction rule and a multimodal intervention approach: a case series. J Othop Sports Phys Ther. 2006;36(3):152–159. | ||
Apelby-Albrecht M, Andersson L, Kleiva IW, Kvåle K, Skillgate E, Josephson A. Concordance of upper limb neurodynamic tests with medical examination and magnetic resonance imaging in patients with cervical radiculopathy: a diagnostic cohort study. J Manipulative Physiol Ther. 2013;36(9):626–632. | ||
Caridi JM, Pumberger M, Hughes AP. Cervical radiculopathy: a review. HSS J. 2011;7(3):265–272. | ||
Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15 Suppl 2:ii1–ii56. | ||
Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol. 2011; 102(2):303–310. | ||
Yoneoka Y, Fujii Y, Tanaka R. Growth of incidental meningiomas. Acta Neurochir (Wien). 2000;142(5):507–511. | ||
Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–1543. | ||
Liu Z, Wang C, Wang H, Wang Y, Li JY, Liu Y. Clinical characteristics and treatment of angiomatous meningiomas: a report of 27 cases. Int J Clin Exp Pathol. 2013;6(4):695–702. | ||
Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011;16(11):1604–1613. | ||
Cushing H, Eisenhardt L. Meningiomas. Their classification, regional behaviour, life history and surgical end results. Bull Med Libr Assoc. 1938;27(2):185. | ||
Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58(1):51–56. | ||
Khalatbari M, Ghalenoui H, Yahyavi ST, Borghei-Razavi H. Left somatosensory cortex tumor presented with radicular hand pain and paresthesia. Arch Iran Med. 2008;11(1):107–109. | ||
Jellad A, Ben Salah Z, Boudokhane S, Migaou H, Bahri I, Rejeb N. The value of intermittent cervical traction in recent cervical radiculopathy. Ann Phys Rehabil Med. 2009;52(9):638–652. | ||
Schott G. Penfield’s homunculus: a note on cerebral cartography. J Neurol Neurosurg Psychiatry. 1993;56:329–333. | ||
Dillin W, Booth R, Cuckler J, Balderston R, Simeone F, Rothman R. Cervical radiculopathy: a review. Spine (Phila Pa 1976). 1986;11(10): 988–991. | ||
Gorter K. Influence of laminectomy on the course of cervical myelopathy. Acta Neurochir (Wien). 1976;33(3–4):265–281. | ||
Lunsford LD, Bissonette DJ, Jannetta PJ, Sheptak PE, Zorub DS. Anterior surgery for cervical disc disease. Part 1: Treatment of lateral cervical disc herniation in 253 cases. J Neurosurg. 1980;53(1):1–11. | ||
Walter CA. Histological studies on the localisation of cerebral function. Br J Psychiatry. 1904;50(211):651–662. | ||
Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976). 1986;11(6):521–524. | ||
Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80(1):19–24. | ||
Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409–421. | ||
Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69–73. | ||
Wainner RS, Gill H. Diagnosis and nonoperative management of cervical radiculopathy. J Orthop Sports Phys Ther. 2000;30(12):728–744. | ||
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114(2):97–109. | ||
Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56–60; discussion 60. | ||
Komotar RJ, Iorgulescu JB, Raper DM, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012;117(4):679–686. | ||
Detti B, Scoccianti S, Di Cataldo V, et al. Atypical and malignant meningioma: outcome and prognostic factors in 68 irradiated patients. J Neurooncol. 2013;115(3):421–427. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.