Back to Journals » OncoTargets and Therapy » Volume 8
Bortezomib-based vs non-bortezomib-based post-transplantation treatment in multiple myeloma patients: a systematic review and meta-analysis of Phase III randomized controlled trials
Authors Liu X, He CK, Meng X, He L, Li K, Liang Q, Shao L, Liu S
Received 17 March 2015
Accepted for publication 22 April 2015
Published 15 June 2015 Volume 2015:8 Pages 1459—1469
DOI https://doi.org/10.2147/OTT.S84828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Daniele Santini
Xiaoping Liu,1 Colin K He,2 Xiangyu Meng,1 Li He,1 Kaili Li,1 Qing Liang,1 Liang Shao,1 Shangqin Liu1
1Department of Hematology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China; 2Orient Health Care, NYC, USA
Objective: To evaluate the efficacy and safety of bortezomib-based vs non-bortezomib-based post-transplantation therapy in patients with multiple myeloma.
Methods: Data of relevant randomized controlled trials assessing the effect of bortezomib as post-transplantation consolidation or maintenance therapy was obtained through a comprehensive search. The outcome measures included response rate, progression-free survival, overall survival, and adverse events (AEs). The hazard ratio (HR), Cochran-Mantel-Haenszel odds ratio (OR), and 95% confidence interval (95% CI) were applied to evaluate the effect of bortezomib in relation to the end points such as progression-free survival, overall survival, response rate, and AEs.
Results: Three randomized controlled trials comprising 1,518 participants were included in this study. Pooled ORs for the rates of overall response, and complete response and near complete response, were 1.85 and 1.75, respectively. Pooled HR for progression-free survival favored bortezomib-based therapy over non-bortezomib-based therapy (0.73, 95% CI: 0.67–0.81), while no statistically significant difference could be found between the two groups regarding the pooled HR for 3-year overall survival. Moreover, incidence rates of overall adverse events and grade 3 and 4 peripheral neuropathy were similar in the bortezomib-based groups and the non-bortezomib-based groups (P=0.12 and P=0.41, respectively). The corresponding cumulative meta-analyses of the rates of overall response rate, complete response and near complete response, and grades 3 and 4 peripheral neuropathy supported the superiority of bortezomib-based maintenance therapy over consolidation therapy.
Conclusion: Bortezomib-based therapy after autologous stem cell transplantation, with tolerable AEs, could obviously improve the response as well as the outcome of multiple myeloma patients, particularly when bortezomib was administered as maintenance therapy.
Keywords: multiple myeloma, post-transplantation therapy, bortezomib-based regimen
Introduction
Multiple myeloma (MM), a common hematological malignancy originates from defects in plasma cells and accounts for 20% of all deaths caused by hematological malignancies.1 Although the clinical application of high-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) significantly improves the survival rate in newly diagnosed MM, the clinical relapse after a certain period of remission is still inevitable in most patients.2 Therefore, post-transplantation therapy such as consolidation (2–4 cycles of combination therapies) and/or maintenance (continuous therapy, usually with single agents, until disease progression) is considered as a promising strategy for achieving durable remission and preventing tumor progression.
Several randomized controlled trials (RCTs) have evaluated the outcome of post-transplantation therapy. Administration of interferon-α was associated with marginally beneficial effects on progression-free survival (PFS) and overall survival (OS). However, due to toxic side effects and poor tolerance, interferon-α maintenance therapy after transplantation is rarely used nowadays.3 Although maintenance therapy with corticosteroid has prolonged PFS, its effect on OS remains controversial.4,5 Immunomodulatory drugs such as thalidomide and lenalidomide, which have been studied as maintenance therapy during the post-transplantation period, have shown improvements, particularly in terms of PFS, while their effects on OS are still debatable.6–8 In addition, a recent study demonstrated that maintenance therapy with lenalidomide could impair the thymic T-cell reconstitution, probably jeopardizing the immunological surveillance over a long period of follow-up.9
Bortezomib is a selective and reversible proteasome inhibitor, which is associated with a high response rate observed both in newly diagnosed MM patients and patients with relapsed or refractory MM.10 It has been used in the consolidation or maintenance therapy for patients who have previously undergone ASCT in several RCTs,11–13 where bortezomib-based therapy after ASCT was compared with non-bortezomib-based therapy; these included trials NCT001134484, NCT00417911, and HOVON-65/GMMG-HD4, in which bortezomib-thalidomide-dexamethasone was compared with thalidomide-dexamethasone (VTD vs TD); bortezomib with placebo; and bortezomib-doxorubicin-dexamethasone with vincristine-doxorubicin-dexamethasone (VAD), respectively. Each study met its primary objective, in which significant improvements in response rates as well as substantial improvements in PFS/time to progression (TTP) were consistently demonstrated, favoring bortezomib-based against non-bortezomib-based post-transplantation therapy. Therefore, we conducted a meta-analysis of data from these Phase III studies to characterize the overall effect of bortezomib-based vs non-bortezomib-based post-transplantation therapy. Complete response (CR) and/or near complete response (nCR) rate, PFS, and OS were the three key end points during post-transplantation treatments.
Methods
Data sources
The data were obtained by searching databases for published, unpublished, and ongoing trials. The databases included PubMed, Embase, the Cochrane Library and the Science Citation Index, and other relevant websites (eg, http://www.controlledtrials.com/, http://www.clinicaltrials.gov/ct). Data from conference proceedings of the American Society of Hematology (2000–2014), the American Society of Clinical Oncology (2000–2014), and European Hematology Association was also collected. The key words utilized were “multiple myeloma OR plasmacytoma”, “ASCT OR autologous stem-cell transplantation OR BMT OR bone marrow transplantation”, “maintenance OR consolidation”, and “bortezomib OR velcade”. Additional potentially relevant studies in the reference lists of the trials were identified and other published systemic reviews and practice guidelines were examined as well.
Study selection
We reviewed all the titles and abstracts obtained from the results of our search strategy to select potential articles. After all full-text papers were reviewed independently by two review authors, the eligibility of these articles was further verified to ensure that they met the inclusion criteria: 1) the studies were RCTs; 2) the participants were patients with newly diagnosed MM of any stage and who had been treated with induction chemotherapies followed by ASCT; 3) intervention was bortezomib-containing regimens; 4) the corresponding control was a placebo or other non-bortezomib-containing regimens; 5) the outcomes reported should include PFS/EFS (event-free survival), OS as well as response rate of CR/nCR, VGPR (very good partial response), and PR (partial response). Multiple reports of the same trial were considered as one study. According to the Jadad scale that includes the reporting of the randomization method, blinding scores, and completeness of follow-up, the maximal score for an included study was 5 and studies were classified on the basis of quality as high (score: 3–5) vs low (score: 0–2).14
Outcome measures
The aim of this meta-analysis is to evaluate the effect of bortezomib as post-ASCT therapy on the survival of newly diagnosed MM patients. The key end points for this review are OS (calculated from the date of randomization until death from any cause), PFS (measured from the date of randomization to the time of disease progression, relapse, or death), and efficacy index (ie, CR/nCR).
Data extraction
Relevant studies were investigated through full-text review, and only those that met all the inclusion criteria were included in the final analysis. A predesigned data extraction form involving baseline characteristics, outcomes, and numbers of events was utilized in the data extraction process which was conducted by Xiaoping Liu and Xiangyu Meng independently. Any discrepancies between the two investigators at the screening or data extraction stage were resolved by discussion.
Statistical analysis
We used meta-package in R 3.1.1 software for all meta-analyses. As to the end points of PFS and OS, the hazard ratio (HR) and 95% confidence interval (95% CI) were applied to evaluate the effect of bortezomib. With respect to comparison of response rates between patients receiving bortezomib-based and non-bortezomib-based consolidation or maintenance therapy, the Cochran–Mantel–Haenszel test was conducted and odds ratios (OR) and 95% CI were calculated; P-values were determined using the chi-square test. In order to show individual study and each cumulative step ratio, traditional meta-analysis and cumulative meta-analysis were performed simultaneously. The I2 statistic was used to quantify heterogeneity among the studies. Any value of I2 less than 25%, 25%–50%, and greater than 50% was defined as low, moderate, and high heterogeneity, respectively. When high heterogeneity was detected, a random-effects model and sensitivity analysis were used for explanation and solution. The publication biases were examined by Egger’s test with which the “trim and fill” method15,16 was used to add several hypothetical studies to the primary meta-analysis to make it unbiased upon detection of bias.
Results
Literature search results
A total number of 2,529 literatures were identified through a comprehensive literature search, which included 315 clinical trials among which nine articles were considered worth of an overall evaluation. After the titles and abstracts were investigated, six articles17–22 were excluded, because two of them were subgroup analysis of two independent Phase III trials, two articles were duplicate reports of two other included articles, one article did not address consolidation or maintenance therapy, and one article was excluded since the key end points of interest (eg, OS, PFS, after consolidation and maintenance treatment) were not reported. Hence, three articles were finally included in this meta-analysis (Figure 1).
  | Figure 1 Flow diagram of study selection. |
Description of included trials
Bortezomib-based regimen was administered as consolidation therapy in two trials11,12 and maintenance treatment in the other trial.13 The methodological quality of each study assessed according to the Jadad scale was shown in Table 1, and the characteristics of the eligible studies were described in Table 2. All patients (n=1,518) in the three studies received ASCT preceded by high-dose therapy (HDT), then the post-transplantation therapy with bortezomib-based or non-bortezomib-based regimens were administered. Five hundred and seventy five patients had an exposure history of bortezomib-based induction therapy, and the remaining 945 patients were naïve to bortezomib-containing treatment (Table 3). Three-hundred and twenty six patients received two ASCTs and 499 patients underwent only one ASCT, while for the rest of the patients the times of ASCT that they had received after induction therapy could not be determined. The median duration of follow-up ranged from 30.4 to 40 months while the median duration of bortezomib treatment varied from 2 to 24 months.
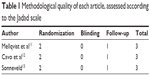  | Table 1 Methodological quality of each article, assessed according to the Jadad scale |
  | Table 2 Patient demographics and baseline disease characteristics |
  | Table 3 Bortezomib-based therapy of the included studies |
Response to treatment
In the three included trials, the response rates of previous treatment after ASCT have been reported for all participants, and no significant difference was found between experimental groups and control groups (P=0.12). As shown in Figure 2A, the Egger’s regression plot (intercept P-value =0.0383<0.05) indicated publication bias (Figure S1). Hence, we used the “trim and fill” method to fill one hypothetical (missing) study to the primary meta-analysis to make it unbiased. After filling, intercept P-value of Egger’s regression was 0.4804>0.05 (Figure S1). The adjusted pooled OR for overall response rate (CR/nCR+VGPR+PR) was 1.85 (95% CI: 1.29–2.64), and the pooled ORs for consolidation and maintenance therapy studies were 1.63 (95% CI: 0.81–3.82) and 1.93 (95% CI: 1.28–2.92), respectively. Moreover, from the cumulative forest plot, OR has an increasing trend as consolidation studies are added. Pooled OR from cumulative analysis of consolidation therapy was 1.63 (95% CI: 0.81–3.82), and no significant difference was found. After adding the maintenance treatment study conducted by Pieter Sonneveld, the OR was larger than 1 (OR =1.85, 95% CI: 1.29–2.64). On the other hand, our integrate analysis demonstrated that the rate of CR/nCR in bortezomib-based groups was significantly higher than that in non-bortezomib-based groups (53.0% vs 39.8%, P<0.001), and the pooled OR for the rates of CR/nCR was 1.75 (95% CI: 1.42–2.15, Figures 2B, S2), and the pooled ORs for consolidation and maintenance therapy studies were 1.62 (95% CI: 1.18–2.22) and 1.86 (95% CI: 1.40–2.46), respectively. Meanwhile, the cumulative meta-analysis indicated that the beneficial effect of bortezomib-based post-transplantation treatment was more obvious when it was administrated as maintenance treatment with more narrow confidence interval (OR =1.75, 95% CI: 1.42–2.15 vs OR =1.62, 95% CI: 1.18–2.22).
Progression-free survival
All the included three trials reported PFS, and the pooled HR for PFS shown in Figure 3A was 0.73 (95% CI: 0.67–0.81), indicating that there was a 27% reduction in the risk of disease progression or death with bortezomib-based therapy after ASCT. No publication bias was detected (Egger’s regression intercept P-value =0.6994>0.05, Figure S3). Moreover, the pooled ORs for consolidation and maintenance therapy studies were 0.73 (95% CI: 0.65–0.81) and 0.75 (95% CI: 0.63–0.90), respectively. Meanwhile, pooled HR from the cumulative meta-analysis for PFS confirmed the beneficial effect of bortezomib-based over non-bortezomib-based post-transplantation therapy.
Overall survival
All the three trials reported 3-year OS, and all the trials claimed that there was no statistical difference between experimental and control groups, which is consistent with our traditional and cumulative meta-analysis (HR for 3-year OS was 0.78, 95% CI: 0.57–1.06, P=0.90; Figure 3B). No publication bias was detected (Egger’s regression intercept P-value =0.9047>0.05, Figure S4), and the pooled HRs for consolidation and maintenance therapy studies were 0.81 (95% CI: 0.53–1.25) and 0.75 (95% CI: 0.48–1.16), respectively.
Adverse events
Meta-analysis of the available data from all the three trials suggested that the frequencies of both overall adverse event (AE) and grades 3 and 4 peripheral neuropathy (PN) were similar in the bortezomib-based groups and the non-bortezomib-based groups (P=0.12 and P=0.41, respectively). Cumulative meta-analysis indicated that there were similar frequencies of grades 3 and 4 PN between bortezomib-based and non-bortezomib-based groups when maintenance treatment was added to the meta-analysis (OR =1.62, 95% CI: 0.73–3.61) (Figure 4), while there was a significant difference between bortezomib-based groups and non-bortezomib-based groups when the cumulative meta-analysis just included the consolidation treatment (OR =4.26, 95% CI: 1.06–17.11). No publication bias was detected (Egger’s regression intercept P-value was 0.619>0.05, Figure S5), and the pooled ORs for consolidation and maintenance therapy studies were 4.26 (95% CI: 1.06–17.11) and 0.73 (95% CI: 0.24–2.27), respectively. Meanwhile, both neutropenia and thrombocytopenia were more frequently observed in bortezomib-based group after ASCT (data not fully shown).
  | Figure 4 Traditional and cumulative meta-analyses of the frequency of grade 3 or 4 peripheral neuropathy. |
Discussion
Although HDT followed by ASCT (HDT–ASCT) has been established as the frontline therapy for young patients with newly diagnosed MM, post-transplantation treatment aimed at enhancing the rate and quality of response achieved in the previous treatment phase (consolidation), and reducing the risk of progression or relapse and to prolong survival period (maintenance) is necessary.23,24 To evaluate the efficacy and safety of bortezomib-based regimen administered as consolidation or maintenance therapy after ASCT, we conducted this meta-analysis of three RCTs.11–13
In our integrated analyses, the rate of CR/nCR (53.0% vs 39.8%) after transplantation was higher in bortezomib-based therapy groups than that in non-bortezomib-based therapy groups, indicating consistent conclusion with previous meta-analysis performed by Sonneveld et al which demonstrated that in the bortezomib-based induction groups, the CR/nCR rate after transplantation was significantly higher compared with that in the non-bortezomib-based induction groups (38% vs 24%, OR =2.05, 95% CI: 1.64–2.56).25 Interestingly, an obvious difference between these two analyses was found, showing that the CR/nCR rates, both in bortezomib-based and non-bortezomib-based therapy groups, were significantly higher after post-transplantation therapy compared with those observed after ASCT. High-quality CR/nCR rates were observed more frequently and obtained more rapidly in previous studies in which bortezomib was incorporated into a multiagent combination regimen for both induction prior to and consolidation after tandem transplant. Hence, we can draw a conclusion that post-transplantation therapy with the goal to enhance the rate and quality of response obtained in the previous treatment phase(s) is well worth applying; Meanwhile, because a total number of 573 (37.7%) participants in the bortezomib-based groups had a history of bortezomib exposure in two RCTs, which might have contributed to the higher CR/nCR rate, we can speculate that the administration of bortezomib into induction, consolidation, and maintenance regimen is worth application in MM treatment. Furthermore, our cumulative meta-analysis confirmed a superiority in CR/nCR and ORR rates when bortezomib-based regimen was administered as a maintenance therapy rather than consolidation therapy ([OR =1.86, 95% CI: 1.40–2.46 vs OR =1.62, 95% CI: 1.18–2.22] and [OR =1.93, 95% CI: 1.28–2.92 vs OR =1.63, 95% CI: 0.81–3.28], respectively), and the difference between consolidation therapy and maintenance therapy could be attributed to an accumulative effect of bortezomib during the longer period of treatment. Based on these results, we conclude that maintenance therapy with bortezomib-containing regimen is worth consideration in order to get higher response rate.
Our integrated analysis suggested that bortezomib-based post-transplantation treatment improved PFS with a pooled HR 0.73 with low heterogeneity (I2 =0%), and our cumulative meta-analysis indicated that both consolidation and maintenance therapy could improve PFS. The benefit of bortezomib-based treatment after ASCT has already been established by several other studies; however, the overall effect of HR demonstrated in our integrated analysis could be introduced as a reference for further investigation of the extent of PFS improvement in future studies. Meanwhile, as stated earlier, no significant difference can be found in OS between bortezomib-based and non-bortezomib-based groups (P=0.90). Relatively short follow-up time, highly effective salvage therapy after disease progression, and a median OS estimate of 7–8 years in young newly diagnosed MM patients eligible for transplantation might have contributed to the similarity in OS between experimental and control groups. Additionally, in a Phase III trial of lenalidomide plus dexamethasone, an improved OS was observed during prolonged follow-up.26 Thus, an evident OS benefit might be confirmed in long-term follow-up.
With regard to the safety profile and toxicities, our study indicated that the rates of overall AEs and drug-related deaths or discontinuation from follow-up were similar in experimental and control groups. As stated earlier, similar frequencies of overall AEs and grades 3 and 4 PN were observed both in bortezomib-based and non-bortezomib-based groups. Cumulative meta-analysis showed that grades 3 and 4 PN was more frequent in bortezomib-containing consolidation therapy groups (with the duration of bortezomib being less than 6 months); however, no statistical significance was found for differences between the experimental and control groups when the maintenance treatment was added to the meta-analysis. This is in accordance with the result of a Phase III APEX trial in relapsed MM demonstrating that the neuropathy generally occurs in the first five cycles of bortezomib treatment, which is related to the accumulated dose; after the fifth cycle (with an accumulated dose of approximately 30 mg/m2), the incidence of neuropathy reaches a plateau, and increases since then by only 4% until the eighth and final cycle.27 Moreover, administration adjustment of bortezomib, such as dose reduction, once weekly regimen,28 and subcutaneous administration,29,30 might help to reduce the incidence and severity of neuropathy. Therefore, the results in the present study indicated that bortezomib-based post-transplantation treatment, especially maintenance, was well tolerated and the treatment-related risks did not appear to outweigh the benefits of treatment.
In recent years, some clinical trials (including the analyzed three RCTs) showed that the benefits of bortezomib consolidation and maintenance were more evident in patients who had not previously achieved at least VGPR;11 patients who achieved CR/nCR after their induction therapy have a tendency of getting better outcomes in terms of PFS and OS;31–33 cytogenetic abnormalities, such as t(4;14) and del(17p), might more or less influence the response and outcome of bortezomib treatment;34,35 however, due to lack of patient-level data, such associations could not be confirmed in this meta-analysis. In addition, the difference in patient characteristics, induction regimens, and the frequency of transplantation between the two groups could be potential confounding factors interfering with the interpretation of our studies. Therefore, more qualified data or RCTs are required to confirm our analysis.
In conclusion, post-transplantation therapy (especially maintenance therapy) with bortezomib-based regimen contributes to improved response rate and PFS with a favorable safety profile. However, prolonged follow-up period is required to confirm the beneficial effect of bortezomib-based post-transplantation therapy conferred on OS.
Acknowledgment
This work was supported by the Research Fund from the National Natural Science Foundation of China (No 81272627).
Disclosure
The authors report no conflicts of interest.
References
Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87. | ||
Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med. 2009;360(25):2645–2654. | ||
Schaar CG, Kluin-Nelemans HC, Te Marvelde C, et al. Interferon-alpha as maintenance therapy in patients with multiple myeloma. Ann Oncol. 2005;16(4):634–639. | ||
Chou T, Tobinai K, Uike N, et al. Melphalan-prednisolone and vincristine-doxorubicin-dexamethasone chemotherapy followed by prednisolone/interferon maintenance therapy for multiple myeloma: Japan Clinical Oncology Group Study, JCOG0112. Jpn J Clin Oncol. 2011;41(4):586–589. | ||
Berenson JR, Crowley JJ, Grogan TM, et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood. 2002;99(9):3163–3168. | ||
Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7–15. | ||
Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28(5):800–807. | ||
Ye X, Huang J, Pan Q. Maintenance therapy with immunomodulatory drugs after autologous stem cell transplantation in patients with multiple myeloma: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e72635. | ||
Clave E, Douay C, Coman T, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T-cell homeostasis. Leuk Lymphoma. 2014;55(8):1788–1795. | ||
Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10(5):361–369. | ||
Mellqvist UH, Gimsing P, Hjertner O, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized Phase 3 trial. Blood. 2013;121(23):4647–4654. | ||
Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120(1):9–19. | ||
Sonneveld P, Schmidt-Wolf IG, van der Holt B. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized Phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Duval S, Tweedie R. Trim and Fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics.2000;56(2):455–463. | ||
Duval S, Tweedie R. A nonparametric “Trim and Fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000; 95(449):89–98. DOI: 10.1080/01621459.2000.10473905. | ||
Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working arty of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30(20):2475–2482. | ||
Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–154. | ||
Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized Phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. | ||
Kim HJ, Yoon SS, Lee DS, et al. Sequential vincristine, adriamycin, dexamethasone (VAD) followed by bortezomib, thalidomide, dexamethasone (VTD) as induction, followed by high-dose therapy with autologous stem cell transplant and consolidation therapy with bortezomib for newly diagnosed multiple myeloma: results of a Phase II trial. Ann Hematol. 2012;91(2):249–256. | ||
Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. | ||
Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised Phase 3 study. Lancet. 2010;376(9758):2075–2085. | ||
Palumbo A, Mina R, Cerrato C, et al. Role of consolidation/maintenance therapy in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2013;13 (Suppl 2):S349–S354. | ||
Cavo M, Brioli A, Tacchetti P, et al. Role of consolidation therapy in transplant eligible multiple myeloma patients. Semin Oncol. 2013;40(5):610–617. | ||
Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of Phase III randomized, controlled trials. J Clin Oncol. 2013;31(26):3279–3287. | ||
Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 Phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152. | ||
Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the Phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. | ||
Wang Y, Ai L, Cui G, et al. Once- versus twice-weekly Bortezomib induction therapy with dexamethasone in newly diagnosed multiple myeloma. J Huazhong Univ Sci Technolog Med Sci. 2012;32(4):495–500. | ||
Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet. 2012;51(12):823–829. | ||
Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, Phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. | ||
Lonial S and Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28(2):258–268. | ||
Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28(15):2612–2624. | ||
van de Velde HJ, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399–1406. | ||
Biran N, Jagannath S, Chari A. Risk stratification in multiple myeloma, part 2: the significance of genetic risk factors in the era of currently available therapies. Clin Adv Hematol Oncol. 2013;11(9):578–583. | ||
Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634. |
Supplementary materials
  | Figure S3 Egger’s regression plot for the meta-analysis of end point PFS. |
  | Figure S4 Egger’s regression plot for the meta-analysis of end point OS. |
  | Figure S5 Egger’s regression plot for the meta-analysis of end point overall adverse events. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




