Back to Journals » International Journal of Women's Health » Volume 7
Benefits, risks, and safety of external beam radiation therapy for breast cancer
Authors Brown L, Mutter R, Halyard M
Received 14 November 2014
Accepted for publication 24 December 2014
Published 24 April 2015 Volume 2015:7 Pages 449—458
DOI https://doi.org/10.2147/IJWH.S55552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Lindsay C Brown,1 Robert W Mutter,1 Michele Y Halyard2
1Department of Radiation Oncology, Mayo Clinic, Rochester, MN, 2Department of Radiation Oncology, Mayo Clinic, Scottsdale, AZ, USA
Abstract: Breast cancer is a common and complex disease often necessitating multimodality care. Breast cancer may be treated with surgical resection, radiotherapy (RT), and systemic therapy, including chemotherapy, hormonal therapy, and targeted therapies, or a combination thereof. In the past 50 years, RT has played an increasingly significant role in the treatment of breast cancer, resulting in improvements in locoregional control and survival for women undergoing mastectomy who are at high risk of recurrence, and allowing for breast conservation in certain settings. Although radiation provides significant benefit to many women with breast cancer, it is also associated with risks of toxicity, including cardiac and pulmonary toxicity, lymphedema, and secondary malignancy. RT techniques have advanced and continue to evolve dramatically, offering increased precision and reproducibility of treatment delivery and flexibility of treatment schedule. This increased sophistication of RT offers promise of improved outcomes by maintaining or improving efficacy, reducing toxicity, and increasing patient access and convenience. A review of the role of radiation therapy in breast cancer, its associated toxicities and efforts in toxicity reduction is presented.
Keywords: breast malignancy, radiotherapy, toxicity, outcomes
Introduction
Breast cancer is the most common malignancy affecting women, with approximately 1.7 million cases diagnosed worldwide in 2012 and more than 500,000 deaths resulting from the disease.1 Thus, continued improvements in the management of breast cancer have a broad and far-reaching impact. This review will give an overview of the benefits of radiotherapy (RT) for breast cancer, including a discussion of RT in the postmastectomy and in the breast-conserving therapy (BCT) settings. Various treatment delivery strategies and their respective benefits and drawbacks are presented. Toxicities associated with RT for breast cancer and recent improvements in treatment planning and delivery, which continue to result in toxicity reduction, are discussed. Although primarily focused on RT, the review begins with a brief summary of the evolution of surgery for breast cancer, which laid the groundwork for later incorporation of RT as a component of breast cancer management.
Evolution of surgical techniques: from Halsted mastectomy to breast conservation
The first revolution in the management of breast cancer was the development of radical mastectomy (RM) by Dr William Halsted in the late 1800s.2 This technique led to significant improvement in the survival of women with breast cancer and, with minor modifications, was considered the standard of curative care for nearly a century thereafter. Although oncologically effective, the procedure was morbid and disfiguring due to removal of the pectoralis major and minor muscles in addition to the breast and the axillary lymph nodes,2 often resulting in significant and lasting pain and dysfunction.
With time, investigators began to question whether such a radical and morbid surgery was necessary for all breast cancer patients.3 To investigate the possibility of surgical de-escalation, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 study was designed, in which women with clinically node-negative operable breast cancer were assigned to RM, total mastectomy (TM; removal of breast tissue without removal of the pectoralis musculature and without dissection of the axilla) with regional RT (designed to include the chest wall and axillary lymph nodes, supraclavicular lymph nodes, and internal mammary lymph nodes [IMN]), or TM alone followed by axillary dissection for those who subsequently developed clinically positive nodes. Women with clinically node-positive disease at diagnosis were randomized to RM or TM with regional RT.4 With 25 years of follow-up, rates of locoregional recurrence (LRR) for clinically node-negative women were lowest in the TM with regional RT arm; for women with node-positive disease, LRR was not significantly different between the two arms. No significant differences in disease-free survival (DFS) or overall survival (OS) were observed between treatment arms in either group.5 With these data, modified RM (MRM; TM plus axillary dissection) became the mastectomy procedure of choice, and the ability of RT to enable less aggressive surgery was established.
With hopes of allowing well-selected breast cancer patients to avoid need for mastectomy in any form, a landmark trial was developed in Milan in 1969 in order to evaluate the potential effectiveness of BCT.6 The trial randomized clinical T1N0 breast cancer patients to RM or quadrantectomy with axillary dissection and adjuvant RT to the conserved breast. Although the risk of local recurrence was significantly higher in women treated with quadrantectomy plus RT (termed breast-conserving therapy, indicating surgical removal of the cancer while preserving the remaining breast, BCT), at 8.8% versus 2.3% at 20 years, the majority of BCT patients with recurrence underwent successful salvage mastectomy, and thus, there was no difference in breast cancer mortality or in death from all causes.
A similar trial, NSABP B-06, was conducted in the United States, in which women with tumors 4 cm or less in diameter were treated with lumpectomy (surgical extirpation of tumor and sufficient surrounding normal tissues to achieve negative surgical margins) alone, lumpectomy plus RT, or TM.7 In addition to confirming equivalency in survival between the groups, consistent with the results from the Italian study, NSABP B-06 analyzed the benefit of the addition of RT to lumpectomy. At 20 years, the cumulative incidence of recurrence in the ipsilateral breast after lumpectomy alone was 39.2%; after lumpectomy plus RT, recurrence was 14.3%. Four subsequent trials confirmed the equivalence in survival of BCT (lumpectomy with adjuvant RT) and mastectomy.8–11 Given this robust body of data, the National Institutes of Health published a consensus statement in 1991, declaring that BCT is an “appropriate method of primary therapy for the majority of women with stage I and II breast cancer and is preferable because it provides survival rates equivalent to those of TM and axillary dissection while preserving the breast.”12 For women with locally advanced disease, mastectomy remained the primary treatment modality.
Radiotherapy in the postmastectomy setting
Although the aforementioned trials established breast conservation as a viable treatment strategy for women with early stage breast cancer, patients with certain specific clinical characteristics are best managed with mastectomy. Possible contraindications to BCT include pregnancy, multicentric disease (cancer in multiple quadrants of the breast), history of prior RT to the affected region, inability to obtain negative surgical margins, anticipated poor cosmetic outcome due to high tumor size to breast size ratio, and the presence of contraindications for RT (such as severe collagen vascular disease). In addition, mastectomy remains the standard surgical procedure for patients with locally advanced disease.13 Finally, some BCT-eligible patients with early stage breast cancer choose to have mastectomy.
Certain women who undergo MRM remain at significant risk of locoregional failure and benefit from postmastectomy radiation therapy (PMRT). In the late 1970s and 1980s, three landmark trials evaluated the benefit of PMRT. The first, performed in British Columbia, Canada, included premenopausal women who had undergone MRM and were found to have one or more positive axillary lymph nodes. Patients were randomized to adjuvant chemotherapy alone or to adjuvant chemotherapy plus RT (37.5 Gy delivered in 16 fractions) to the chest wall and regional lymph nodes (axillary, supraclavicular, and IMN). At 20 years of follow-up, the addition of PMRT resulted in improvement in all oncologic outcomes, including locoregional and distant recurrence-free survival and OS.14 The Danish Breast Cancer Group performed two additional studies evaluating the utility of PMRT in premenopausal (protocol 82b)15 and postmenopausal (protocol 82c)16 women. Eligible patients included women who had undergone MRM for non-metastatic disease with one or more positive axillary lymph nodes, tumor size greater than 5 cm, or skin or pectoral fascia invasion. Premenopausal women (82b) received chemotherapy, and postmenopausal women (82c) received tamoxifen, and all were randomized to receive RT to the chest wall and regional lymph nodes or no RT. Radiation doses prescribed were 50 Gy in 25 fractions or 48 Gy in 22 fractions. Consistent with the British Columbia results, the addition of RT improved all endpoints, including local and distant control and OS.15–17 The 10-year absolute survival benefit with the addition of PMRT to systemic therapy was remarkably similar across all three studies, at 9%–10%.
Despite these compelling findings, the indications for PMRT remained controversial. Many felt that the majority of women who met the eligibility criteria for these three seminal studies should be offered PMRT. In contrast, others argued that the survival benefit was likely a result of treatment of the subset of patients with the highest likelihood of LRR, those with the highest nodal burden. For women with four or more positive axillary nodes, it was generally accepted that PMRT should be recommended to improve locoregional control and survival.18 However, the benefit in women with negative nodes or one to three positive axillary lymph nodes remained unclear and the topic of much debate. A recent meta-analysis including more than 8,000 women treated on 22 randomized trials aimed to address the issue of benefit of PMRT for women with zero to three positive nodes.19 The authors found that PMRT did not improve locoregional control or survival in the 700 node-negative women who underwent axillary dissection. However, for the 1,314 women with one to three positive nodes who underwent axillary dissection, PMRT significantly reduced the risk of LRR and breast cancer mortality. This finding held true for women treated with systemic therapy, and the benefit of PMRT appeared to be of the same magnitude whether a patient had one, two, or three positive lymph nodes.
Currently, PMRT is routinely recommended for women with four or more positive axillary lymph nodes and for those with advanced T stage. For women with one to three positive lymph nodes, PMRT should be strongly considered. Decision-making should take into account the presence of other negative prognostic factors, including young age,20–22 triple-negative breast cancer,23,24 extranodal extension of disease,25–28 evidence of angiolymphatic invasion,22,29 large primary tumor,20,25,30,31 high percentage of involved nodes,26,30,32 and close or positive margins.28,29 Potential benefit of treatment must be weighed against possible toxicity, as discussed later in this review. RT fields are typically designed to include the chest wall and supraclavicular lymph nodes. The aforementioned PMRT trials included IMN treatment, which generally results in a higher incidental dose to the heart and lungs; however, the contribution of IMN radiation to survival is unclear, and, thus, inclusion of the IMN in the radiation fields is controversial.33,34
RT as a component of BCT
As discussed above, early trials such as the Milan trial6 and NSABP B-067 established BCT as a viable option for many women with early stage breast cancer. The benefit of adjuvant RT as a component of BCT has been extensively studied. Following breast-conserving surgery for invasive breast cancer, RT to the breast reduces the risk of recurrence and death. The Early Breast Cancer Trial Collaborative Group35 demonstrated that the addition of radiation reduced the 10-year risk of LRR from 25% to 8% and resulted in an absolute reduction in all-cause mortality of 3.8%–5.4% at 15 years.35,36
While RT to the breast reduces in-breast failure, whole breast RT does not comprehensively encompass all regional nodal volumes (axillary, supraclavicular, and IMN). Nodal recurrences contribute significantly to locoregional failure, and thus, the indications for and utility of regional nodal RT, in addition to radiation to the conserved breast, must also be considered.
Regional nodal irradiation (RNI), that is, RT to the axillary, supraclavicular, and IMN basins, is generally indicated in women with four or more positive nodes. As in the postmastectomy setting, the value of RNI in women with one to three positive nodes is controversial. Two recent trials have evaluated the possible benefit of RNI in addition to whole breast radiation following lumpectomy. NCIC-CTG MA.2037 included primarily women with node-positive disease and a smaller number of women with high-risk node-negative disease (10% of the population; defined as those with primary tumor ≥5 cm or ≥2 cm and <10 axillary nodes removed with estrogen receptor negative disease, grade 3 disease, or lymphovascular invasion) who had undergone lumpectomy and axillary surgery. All patients received RT to the breast and were randomized to receive or not receive RNI to the IMN, supraclavicular and axillary nodes. Most (85%) patients had one to three positive lymph nodes and received chemotherapy (91%). The addition of RNI resulted in improved 5-year locoregional DFS (96.8% vs 94.5%, P=0.02) and distant DFS (92.4% vs 87%, P=0.002). A non-significant trend toward improved OS was also seen (92.3% vs 90.7% at 5 years, P=0.07). The European Organisation for Research and Treatment of Cancer performed a similar trial in which patients with medially located tumors and/or involved axillary lymph nodes were randomized to receive or not receive radiation to the IMN and medial supraclavicular lymph nodes.38 The addition of RNI in this trial resulted in significant improvements in 10-year metastasis-free survival (78.0% vs 75.0%, P=0.02) and DFS (72.1% vs 69.1%, P=0.044), and a non-statistically significant improvement in OS (82.3% vs 80.7%, P=0.056).
Given the above data, it is recommended that virtually all patients who have lumpectomy receive RT to the whole breast. Additional RT to the regional lymph nodes should be considered in node-positive patients. The oncology community awaits publication of the full manuscripts of the above-discussed studies, which are expected to add significantly to the understanding of which patients will benefit most from RNI.
Modification of treatment schedules
Despite the equivalence in survival, many women eligible for BCT are treated with mastectomy and, despite the benefits of adjuvant radiation following lumpectomy, many women do not undergo radiation.39,40 These trends are felt to be at least in part due to issues of access to RT and inconvenience associated with protracted treatment regimens.38 Investigators have studied various methods of shortening RT to increase cost-effectiveness and treatment convenience.
Hypofractionation, in which a larger dose of radiation is delivered with each treatment, thereby reducing the total number of treatment days, has been evaluated in several large randomized controlled trials comparing 13 to 16 daily treatments to conventionally fractionated radiation, consisting of 25 daily treatments. With long-term follow-up, these studies have found equivalent rates of local/locoregional control.41,42 Toxicity and cosmetic outcomes have been found to be equivalent or improved with the hypofractionated schedule.41,42 Therefore, hypofractionated protocols are now encouraged for many women with early stage breast cancer undergoing RT to the breast alone.43 When RNI is delivered, many oncologists prefer conventional irradiation, as evidence for hypofractionation with RNI is less robust.
A second strategy to improve convenience of adjuvant RT in the setting of BCT is accelerated partial breast irradiation (APBI). The majority of ipsilateral breast tumor recurrences after lumpectomy without radiation occur in the tumor bed.44 In APBI, RT is delivered only to the tumor bed and the immediate surrounding tissue. This has the benefit of exposing less normal tissue to radiation, thereby potentially decreasing toxicity. Furthermore, with less tissue treated, higher doses per treatment can theoretically be administered.
Various APBI regimens have been investigated, ranging from a single day to 1–2 weeks, and many methods of delivering APBI exist.45 The oldest method with the longest follow-up is multicatheter interstitial brachytherapy, in which multiple flexible after-loading catheters are placed percutaneously into the tumor bed several weeks after surgery.46 Single lumen and multilumen balloon-based and other bundled catheter devices, which are placed in the lumpectomy cavity (intracavitary) at the time of surgical resection or in a second procedure in the days following surgery, are more commonly used in modern-day practice for breast brachytherapy. Intraoperative methods of radiation delivery to the lumpectomy cavity are being evaluated, in which radiation is typically delivered to the tumor bed during the lumpectomy procedure in a single fraction, potentially improving patient convenience by negating the need for later fractionated postoperative radiation.47,48 Lastly, external beam RT has been used to deliver APBI, and involves the non-invasive delivery of radiation to the lumpectomy cavity using similar techniques as in the delivery of whole breast irradiation.
Early results of randomized trials of APBI suggest no survival detriment with use of APBI versus whole breast RT, but a possible detriment in locoregional control, specifically with use of intraoperative radiation techniques.47–49 Long-term results of randomized studies comparing various APBI techniques to whole breast RT are pending. The available data suggest that patients with low-risk features (ie, older patients with small tumors, without nodal disease, who have undergone surgery with negative margins and whose tumors have a favorable phenotype) have reasonable local control after APBI, and guidelines have been published regarding which candidates are currently felt to be appropriate for consideration of APBI outside of a clinical trial.50 The optimal delivery technique, dose, and fractionation remain areas of investigation. While APBI has generally been associated with acceptable toxicity and excellent cosmesis,51 several investigators have reported increased toxicity and poor cosmesis relative to whole breast radiation, particularly when external beam techniques are employed.52,53 Long-term follow-up from randomized trials, such as the NSABP B39/RTOG 0413, will improve our understanding of the locoregional control and adverse effects associated with various delivery methods of APBI and will more clearly define optimal patient selection.
Toxicity associated with breast cancer RT
Although adjuvant RT for breast cancer has been shown to improve locoregional control and OS, a portion of the breast cancer-specific mortality benefit seen with adjuvant RT for breast cancer is offset by radiation-associated morbidity and mortality.54 This toxicity is critical for oncologists to recognize, quantify, and reduce. Specific toxicities that will be addressed in this section are cardiac toxicity, secondary malignancy, radiation pneumonitis, and lymphedema.
Radiation toxicity: cardiac disease
Older data have shown dramatic increases in cardiac mortality following adjuvant RT for left-sided breast cancer. In the aforementioned 2005 meta-analysis from the Early Breast Cancer Trial Collaborative Group,36 the ratio of non-breast cancer deaths in irradiated versus unirradiated patients was 1.12. This excess mortality was multifactorial, but due in large part to an increased risk of death as a result of heart disease in irradiated patients, with a ratio of rates of 1.27 when compared to patients not undergoing RT.
In an effort to better define radiation-associated cardiac toxicity, Dr Sarah Darby and colleagues published a study in 2005 in which they compared cardiac mortality for women who received radiation for left- versus right-sided breast cancer, and found that radiation for left-sided breast cancer increased the risk of cardiac disease and subsequent death.54 In 2013, Darby et al published a case-controlled study analyzing the risk of major coronary events (myocardial infarction, coronary revascularization, or death from ischemic heart disease) and again found that women with left-sided breast cancer had more major coronary events than those treated for right-sided breast cancer.55 The absolute risk of future coronary events and death from cardiac disease was found to correlate with the mean radiation dose to the heart. Interestingly, contrary to the previously widely held belief that cardiac toxicity was a late radiation-associated toxicity, it was found that elevated cardiac risk was apparent within fewer than 5 years of receipt of left-sided RT. Absolute risk of cardiac disease and death was also found to depend heavily upon the presence of preexisting ischemic heart disease and cardiac risk factors, suggesting that minimization of cardiac radiation in patients with elevated cardiac risk at baseline is of greatest absolute benefit and underscoring the importance of cardiac disease risk factor reduction in patients receiving radiation. It is thought that cardiac toxicity associated with breast cancer RT is due, at least in part, to macrovascular damage, particularly to the left anterior descending (LAD) artery,56 which courses along the anterolateral heart border and is often within or near the RT tangential fields used to treat the breast or chest wall in patients with left-sided disease.
It is important to recognize the context of these findings and the vast differences between the RT delivered in past decades versus that delivered today. In the past, RT to the breast or chest wall was delivered with tangential fields designed on 2D films and based on bony anatomy, without the benefit of the cross-sectional imaging routinely used in modern treatment planning. To ensure adequate target coverage, tangents were often deep, traversing much of the anterolateral heart. Furthermore, when now-considered-antiquated treatment delivery methods such as Cobalt-60 were utilized, significant heterogeneity of dose occurred, introducing the possibility of portions of the heart and other normal tissues receiving far more than the prescription dose of radiation.57 With the advent of 3D (computed tomography-based) planning, knowledge of risk of cardiac toxicity and the resulting importance of cardiac sparing, and improved radiation dose distributions owing to adoption of linear accelerators in modern-day radiation oncology, the toxicities associated with treatment are expected to be far reduced with the present-day RT for breast cancer.
Recently, Henson et al quantified the long-term risk of breast cancer-related cardiac toxicity by treatment era.58 For women treated from 1973 to 1982, significantly increased cardiac mortality was seen for those treated for left- versus right-sided breast cancer. Increased risk of cardiac death was evident for women undergoing left-sided treatment in this era within 10 years, and continued to increase with time from RT. Importantly, a similar increase in radiation-related cardiac mortality was seen neither for women treated from 1983 to 1992 nor for those receiving radiation after 1992, suggesting that improvements in RT planning and delivery are translating into reductions in toxicity. Minimization of mean heart dose and dose to the LAD and other coronary arteries in or near the radiation field would be expected to result in further reduction of treatment-associated elevation of cardiac risk.
Radiation toxicity: radiation pneumonitis
Radiation pneumonitis is an inflammatory reaction of lung tissue to radiation, with a spectrum of severity, ranging from asymptomatic pneumonitis incidentally noted on imaging to life-threatening pneumonitis manifest as cough, fever, shortness of breath, and pulmonary failure.59 Although most commonly observed following radiation for intrathoracic tumors, radiation pneumonitis can occur following breast cancer radiation, due to radiation exposure of lung underlying the chest wall, and is related to the amount of lung exposed to radiation and the mean dose to the lung.60 Unlike cardiovascular toxicity, radiation pneumonitis is a subacute manifestation of RT, typically occurring 6 weeks–6 months after radiation, thereby allowing quantification of risk with modern radiation planning and delivery techniques. In the MA.20 trial discussed earlier,37 0.2% of patients randomized to whole breast RT experienced grade 2 pneumonitis (symptomatic, requiring medical intervention), while 1.3% of patients randomized to receive additional radiation to the regional lymph nodes experienced this complication. No patient experienced grade 3 or greater pneumonitis, meaning that they neither had life-threatening pneumonitis nor required oxygen. These numbers suggest that, with modern-day radiation planning and delivery, rates of pneumonitis are low even with comprehensive treatment of the regional lymph nodes, and, when pneumonitis does occur, it tends to be of minimal severity.
Radiation toxicity: lymphedema
Lymphedema of the arm and chest wall is another potential complication of treatment for breast cancer. Although not life-threatening, lymphedema is a potentially debilitating and often irreversible condition that can greatly impact patients’ quality of life. A recent meta-analysis of breast cancer trials found that approximately 20% of women develop lymphedema following breast cancer treatment.61 Risk of lymphedema is most strongly correlated with extent of surgery, with the risk of lymphedema after axillary dissection approximately four times higher than the risk after sentinel lymph node biopsy. Obesity is also a risk factor for lymphedema development.61 It has long been thought that adjuvant RT in any form increased the risk of lymphedema; however, recent data have called this belief into question. There is now significant evidence that radiation to the breast alone does not result in increased lymphedema risk.62,63 Radiation to the regional lymph nodes has been shown to increase risk of lymphedema, compared with radiation to the breast or chest wall alone. A recent publication reported a 3% risk of lymphedema without radiation, 3.1% risk with radiation to the breast or chest wall only, and 21.9% risk with radiation to the supraclavicular nodal basin.63 On multivariate analysis, the addition of supraclavicular radiation significantly increased the lymphedema risk compared with breast/chest wall radiation alone, with an HR of 1.7 (P=0.025). In comparison, the HR for undergoing axillary lymph node dissection was 3.5 (P=0.0001). A recent report of an international trial evaluating axillary lymph node dissection versus axillary RT for women with positive sentinel lymph node biopsy reported reduced lymphedema for patients receiving axillary radiation versus axillary lymph node dissection (10% vs 21% at 5 years).64 Thus, while radiation to the regional lymph nodes increases risk of lymphedema, it appears to do so less than axillary lymph node dissection. With recent data demonstrating the efficacy of sentinel node biopsy alone, even in select node-positive patients, rates of lymphedema are expected to fall. Radiation to the breast or chest wall alone does not contribute to development of lymphedema; however, radiation to the regional nodes dose appear to increase the risk of this condition, and patient counseling and prevention strategies are paramount in women receiving RNI and in those undergoing axillary dissection.
Radiation toxicity: secondary malignancy
It is known that radiation can lead to development of secondary malignancies, and elevated risk of second cancers after radiation for breast cancer has been reported. Although absolute numbers of patients affected are small, increased rates of contralateral breast cancer, lung cancer, esophageal cancer, and soft tissue sarcoma have been documented after RT for breast cancer.36 The relative risk of developing a second solid malignancy in women treated with RT for breast cancer relative to women with breast cancer not treated with radiation is estimated to be 1.1.65 Secondary malignancy following radiation most commonly occurs in areas that directly received radiation, and thus, the best strategy for reduction of secondary malignancy risk is minimization of the integral dose of radiation delivered to tissues, that is, increasing conformality of radiation so that target tissues are adequately treated and nearby normal tissues spared.
Toxicity reduction: improvements in RT planning and delivery
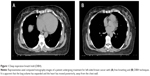
The studies presented earlier in this review regarding cardiac toxicity associated with RT54,57 included data from patients treated as early as the 1950s. Since that time, incredible gains have been achieved in radiation planning and delivery that have improved the tolerability and toxicity of RT. For example, deep inspiration breath hold (DIBH) is a recently developed strategy for further improving sparing of normal structures, specifically the heart and lung.66–68 In conventional radiation planning and delivery, patients breathe freely during acquisition of the planning CT scan and treatment. When DIBH is employed, a planning CT scan is obtained with the patient holding her breath comfortably in deep inspiration. The planning CT is acquired, and the treatment is delivered in 20–30-second increments of breath holding, between which the patient is able to breathe freely and recover. This technique has two possible advantages. First, it expands the lung volume, so that a smaller relative amount of lung is exposed to radiation. Second, in some patients, it causes the heart to be displaced posteriorly, away from the chest wall, so that less heart and LAD coronary artery are in the path of the radiation beam.66 An example of the DIBH technique is provided in Figure 1.
Intensity-modulated radiation therapy (IMRT) is a method of radiation delivery in which multiple beam angles are used and individually modulated to allow for highly conformal radiation delivery. IMRT allows shaping of the dose around critical structures, and is frequently employed in the treatment of cancers in certain sites, such as the head, neck, and pelvis. Use of IMRT for cardiac sparing in breast cancer has been reported.69,70 However, although highly conformal, the technique generally results in larger volumes of lung, heart, and contralateral breast receiving low and intermediate doses of radiation.69 Thus, its use in breast cancer has not been widely adopted.
Proton therapy is another potential strategy for dose optimization in the treatment of breast cancer currently under study.70,71 Unlike conventional photon radiation, in which the beam deposits dose along its entire track through the patient, proton radiation has a low entrance dose, deposits high amounts of energy at a specified location (in the target tissue), and has no exit dose beyond that point. This allows for excellent coverage of target tissues and sparing of normal structures. Dosimetric studies of breast cancer proton therapy appear very promising.70
Lastly, APBI techniques, as previously discussed, result in a lesser volume of normal tissue exposed to radiation. This results in cardiac,72 pulmonary, and lymphatic sparing, and, by reducing the integral dose to tissues, theoretically reduces the risk of secondary malignancy.
Radiation therapy for breast cancer: summary
In summary, the role of RT in the treatment of breast cancer has evolved significantly over the past 50 years. Radiation is a critical component of multimodality treatment for many women, allowing many to conserve their breast without sacrifice in terms of survival, and improving locoregional control and survival likelihood for women with locally advanced disease who undergo mastectomy.
Although there is toxicity associated with radiation for breast cancer, advances in RT planning and delivery have led to reduction in the magnitude of treatment-related morbidity and mortality. Novel treatment strategies such as DIBH and proton therapy have potential to further reduce the risk of late toxicity, improving the therapeutic benefit of radiation for breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed July 24, 2014. | ||
Halsted WS. I. A Clinical and Histological Study of certain Adenocarcinomata of the Breast and a Brief Consideration of the Supraclavicular Operation and of the Results of Operations for Cancer of the Breast from 1889 to 1898 at the Johns Hopkins Hospital. Ann Surg. 1898;28: 557–576. | ||
Keynes G. The place of radium in the treatment of cancer of the breast. Ann Surg. 1937;106:619–630. | ||
Fisher B, Montague E, Redmond C, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer. 1977;39:2827–2839. | ||
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. | ||
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. | ||
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. | ||
Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy. Cancer. 2003;98:697–702. | ||
van Dongen JA, Bartelink H, Fentiman IS, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst. 1992;11:15–18. | ||
Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47:672–681. | ||
Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. | ||
NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. | ||
American College of Radiology. Practice guideline for the breast conservation therapy in the management of invasive breast carcinoma. J Am Coll Surg. 2007;205:362–376. | ||
Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. | ||
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. | ||
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. | ||
Danish Breast Cancer Cooperative Group; Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275. | ||
Recht A, Edge SB, Solin LJ, et al; American Society of Clinical Oncology. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. | ||
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. | ||
Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. | ||
Cheng SH, Tsai SY, Yu BL, et al. Validating a prognostic scoring system for postmastectomy locoregional recurrence in breast cancer. Int J Radiat Oncol Biol Phys. 2013;85:953–958. | ||
Moo TA, McMillan R, Lee M, et al. Selection criteria for postmastectomy radiotherapy in t1-t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20:3169–3174. | ||
Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2858. | ||
Wang J, Shi M, Ling R, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100:200–204. | ||
Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–2827. | ||
Strom EA, Woodward WA, Katz A, et al. Clinical investigation: regional nodal failure patterns in breast cancer patients treated with mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1508–1513. | ||
Tendulkar RD, Rehman S, Shukla ME, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1–3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83:e577–e581. | ||
Woodward WA, Strom EA, Tucker SL, et al. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy: Implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2003;57:336–344. | ||
Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035–1039. | ||
Katz A, Buchholz TA, Thames H, et al. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: implications for adjuvant irradiation. Int J Radiat Oncol Biol Phys. 2001;50:397–403. | ||
Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. | ||
Fortin A, Dagnault A, Blondeau L, Vu TT, Larochelle M. The impact of the number of excised axillary nodes and of the percentage of involved nodes on regional nodal failure in patients treated by breast-conserving surgery with or without regional irradiation. Int J Radiat Oncol Biol Phys. 2006;65:33–39. | ||
Chang JS, Park W, Kim YB, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys. 2013;86:867–872. | ||
Hennequin C, Bossard N, Servagi-Vernat S, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86:860–866. | ||
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. | ||
Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. | ||
Whelan TJ, Ackerman I, Chapman JW, et al. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer.J Clin Oncol. 2011;29: (suppl; abstr LBA1003) ASCO Annual Meeting Proceed (Post-Meeting Edition). | ||
Poortmans P, Struikmans H, Kirkove C, et al. Irradiation of the internal mammary and medial supraclavicular lymph nodes in stage I to III breast cancer: 10 years results of the EORTC radiation oncology and breast cancer groups phase III trial 22922/10925. Oral presentation at: European Cancer Congress; September 28; 2013; Amsterdam, The Netherlands. | ||
Baldwin LM, Taplin SH, Friedman H, Moe R. Access to multidisciplinary cancer care: is it linked to the use of breast-conserving surgery with radiation for early-stage breast carcinoma? Cancer. 2004;100:701–709. | ||
Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. | ||
Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. | ||
Haviland JS, Owen JR, Dewar JA, et al; START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. | ||
choosingwisely.org [homepage on the Internet]. Philadelphia, PA: Choosing Wisely; c.2014. Available from: http://www.choosingwisely.org/wp-content/uploads/2013/09/ASTRO-5things-List_092013.pdf. Accessed November 12, 2014. | ||
Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. | ||
Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90:1–13. | ||
Polgár C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma –5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. | ||
Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. | ||
Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–1277. | ||
Vaidya JS, Wenz F, Bulsara M, et al; TARGIT Trialists’ Group. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–613. | ||
Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74:987–1001. | ||
Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991–999. | ||
Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. | ||
Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. | ||
Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. | ||
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;11:987–998. | ||
Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. | ||
Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–896. | ||
Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. | ||
Rodrigues G, Lock M, D’Souza D, et al. Prediction of radiation pneumonitis by dose – volume histogram parameters in lung cancer – a systematic review. Radiother Oncol. 2004;71:127–138. | ||
Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. | ||
DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. | ||
McCloskey SA, Bandos H, Julian T, et al. The impact of radiation therapy on lymphedema risk and the agreement between subjective and objective lymphedema measures: NSABP B-32 Secondary Data Analysis. Oral Presentation at: Annual Meeting of the American Society for Radiation Oncology; September 14, 2014; San Francisco, CA. | ||
Warren LE, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88:565–571. | ||
Donker M, Rutgers E, van de Velde C, et al. Axillary lymph node dissection vs axillary radiotherapy: A detailed analysis of morbidity. Presented at: Annual Meeting of the American Society of Breast Surgeons; April 30, 2014; Las Vegas, NV. | ||
Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–360. | ||
Korreman SS, Pedersen AN, Aarup LR, Nøttrup TJ, Specht L, Nyström H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2006;65:1375–1380. | ||
Korreman SS, Pedersen AN, Josipović M, et al. Cardiac and pulmonary complication probabilities for breast cancer patients after routine end-inspiration gated radiotherapy. Radiother Oncol. 2006;80:257–262. | ||
Pedersen AN, Korreman S, Nystrom H, et al. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol. 2004;72:53–60. | ||
Coon AB, Dickler A, Kirk MC, et al. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2010;78:104–110. | ||
Johansson J, Isacsson U, Lindman H, Montelius A, Glimelius B. Node-positive left-sided breast cancer patients after breast-conserving surgery: potential outcomes of radiotherapy modalities and techniques. Radiother Oncol. 2002;65:89–98. | ||
MacDonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86:484–490. | ||
Moran JM, Ben-David MA, Marsh RB, et al. Accelerated partial breast irradiation: what is dosimetric effect of advanced technology approaches? Int J Radiat Oncol Biol Phys. 2009;75:294–301. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.