Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 15
Assessment of Nutrients Intake in Pediatrics with Type 1 Diabetes and Dyslipidemia in Jordan
Authors Tayyem R , Nawaiseh H, Zakarneh SB, Khial Y, Allehdan S
Received 7 September 2023
Accepted for publication 4 January 2024
Published 19 March 2024 Volume 2024:15 Pages 31—43
DOI https://doi.org/10.2147/AHMT.S439046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Reema Tayyem,1 Hala Nawaiseh,2 Sara Basem Zakarneh,2 Yasmen Khial,1 Sabika Allehdan3
1Department of Human Nutrition, College of Health Science, Qatar University, Doha, Qatar; 2Department of Nutrition & Food Technology, Faculty of Agriculture, The University of Jordan, Amman, 11942, Jordan; 3Department of Biology, College of Science, University of Bahrain, Zallaq, Kingdom of Bahrain
Correspondence: Reema Tayyem, Email [email protected]
Background: Dyslipidemias are disorders of lipoprotein metabolism that occur during childhood and adolescence, often persist into adulthood, and increase the risk of developing atherosclerotic lesions. This study aimed to assess the potential association between nutrient intake and dyslipidemia in Jordanian pediatric patients diagnosed with type 1 diabetes mellitus.
Methods: This cross-sectional study was conducted in Amman, Jordan, and involved 90 children and adolescents diagnosed with type 1 diabetes mellitus. Caregivers provided the following data: sex, age, type and dose of insulin, age at onset of type 1 diabetes, and level of physical activity. Anthropometric measurements were obtained using calibrated scales, and CDC growth charts were used to assess participants’ body weight status. Nutrient intake was estimated using a 120-item food frequency questionnaire (FFQ) previously validated in Jordanian children and adolescents. Serum lipid levels, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were measured. Binary logistic regression was used to assess the relationship between nutrient intake and dyslipidemia.
Results: The results indicated that 36.7% of study participants had dyslipidemia. There were no significant differences in nutrient intake between dyslipidemic and normolipidemic individuals, except for a significantly higher median intake of vitamin B12 in the dyslipidemic group compared to the normolipidemic group (3.6 versus 2.7 μg, P-value = 0.046).
Conclusion: This study found no significant association between the prevalence of dyslipidemia and nutrient intake in children and adolescents diagnosed with type 1 diabetes mellitus.
Keywords: nutrients, type 1 diabetes, dyslipidemia, children, adolescents
Introduction
The prevalence of dyslipidemia has recently increased in the general population, including children. There is clear evidence that atherosclerotic plaque formation begins in the early years of life and in adolescence, leading to premature adverse cardiovascular problems. According to data from the National Health and Nutrition Examination Survey (NHANES), 20% of participants aged between 12 and 19 years were diagnosed with lipid disorders; however, the prevalence of dyslipidemia among children with obesity was 42% which was higher.1 Negative lifestyle habits, such as sedentism, low physical activity, and poor eating habits, play an important role in being overweight and obese, contributing to an increase in the prevalence of dyslipidemia.2 Dyslipidemia is the elevation of a group of blood lipids such as cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, and high-density lipoprotein cholesterol (HDL-C).3 Dyslipidemia is divided into two types: primary and secondary. Primary dyslipidemia is related to genetics, which means that it is inherited and is caused by a single mutation or multiple mutations that contribute to an elevation in triglyceride and cholesterol.4 Many factors are known to cause secondary dyslipidemia, including diabetes mellitus, cholestatic liver disease, nephrotic syndrome, chronic renal disease, hypothyroidism, obesity, smoking, excessive alcohol consumption, and some medications.5 Patients with type 1 diabetes mellitus (T1DM) are more 2–4 times likely to develop atherosclerosis than are those without diabetes. Moreover, the American Heart Association reported that children with type 1 diabetes have the highest risk of cardiovascular disease.6 In addition, both hyperglycemia and dyslipidemia are common in children and adolescents with T1DM, and most likely increase the risk of developing cardiovascular disease.7
Unhealthy eating habits are a significant risk factor for dyslipidemia.8,9 Diet has a similar effect on serum lipid levels in children and adults.8 Children’s blood lipid and lipoprotein concentrations were correlated with the type and amount of dietary fat consumed.10 It is important to note that no study has investigated the relationship between food intake and dyslipidemia in children in Jordan. Therefore, the purpose of this study was to evaluate nutrient intake and its association with dyslipidemia in pediatric patients diagnosed with T1DM in Jordan.
Methodology
Study Population
This cross-sectional study aimed to assess the nutrient intake of Jordanian children and adolescents with T1DM. Patients in this study were selected from the King Hussein Medical Center (Queen Rania Pediatric Hospital). A total of 90 Jordanian children and adolescents aged between 6 and 18 years who were diagnosed with T1DM from at least 1 to 10 years were selected. The inclusion criteria were an onset of disease of at least 1 year and less than 10 years, children and adolescents aged 6–18 years, insulin use, and Jordanian nationality. The exclusion criteria were patients who suffer from cancer, food allergies, autoimmune diseases, inability to communicate verbally, use of insulin pumps, and newly diagnosed T1DM. Ethical approval, obtained from the Institutional Review Board of the King Hussein Medical Center (Queen Rania Pediatric Hospital) under the number 1/2019/2863, adhered to the ethical standards outlined in the 1975 Declaration of Helsinki. Queen Rania Pediatric Hospital granted the researcher permission to utilize a private room with favorable physical conditions for conducting the interviews. The researcher explained the purpose of the study to all caregivers of the participants and required them to sign a consent form before data collection. All data for each participant in this study had an ID. All information was handled confidentially, and the researcher was the only one with access to the patients’ IDs.
Data Collection
The data were gathered in a hospital setting. King Hussein Medical Center (Queen Rania Pediatric Hospital) provided care for children with T1DM. The outpatient department of the hospital served as the location for the data collection. Data such as sex, age, type, and doses of insulin were obtained from the caregivers of the participants using a personal information questionnaire. Face-to-face interviews with children’s caregivers were conducted for data collection between March 2020 and June 2022. Participants were divided into three groups according to their age into 3 groups, children (aged between 6–8 years), adolescents (aged–9-13), and pre-adolescents (aged–14-18). Additionally, they were divided according to their status as dyslipidemia (dyslipidemia group) or not having dyslipidemia (normolipidemic group).
Physical Activity Questionnaire
Physical activity levels were measured using a questionnaire. Children in grades 4 through 12 were given a general assessment of their level of physical activity using the Physical Activity Questionnaire for Older Children (PAQ-C) and Physical Activity Questionnaire for Adolescents (PAQ-A).11 The questionnaire evaluated the overall level of physical activity.12 These are self-administered, seven-day recall questionnaires that offer inexpensive, easy to administer, valid, and reliable assessment of physical activity for children and adolescents. The survey does not provide an estimate of calorie expenditure; detailed information on frequency, time, and intensity; or a distinction between different activity intensities, such as moderate and intense activities.11 Physical activity was recorded and presented as a score.
Dietary Assessment
The participants’ dietary information was gathered using a validated Arabic food frequency questionnaire (FFQ) with 120 items designed for children and adolescents.13 Participants were asked how frequently, on average, they had eaten each food item over the previous 12 months. Each FFQ question had two parts: a food list that was adjusted to be culturally appropriate for Jordanian children and teenagers’ food items, along with frequency responses for each item and portion size expressed in household measures (such as cups, spoons, and plates) and/or typical packing size. The food list was categorized into multiple groups based on food type. There were 10 options for frequency response: never, ≤ 12 years or less, 2–3 months, 1–2 weeks, 3–4 weeks, 5–6 weeks, 1 day, 2–3 days, 4–5 days, and ≤ 6 days. Foods typically consumed at specific times of the year were considered for seasonal variation. Food consumption frequency was designed to calculate intake throughout the year. Instead of using terms such as fruits, eggs, crackers, and pastries, common units or usual units were used for some foods such as one apple, one egg, and one piece of cracker or pastry. Food models were used to assist the participants in evaluating the consumed portion size of foods that could not be assessed using standard measuring units. Metric measurements in grams or milliliters were recorded for each food category. The FFQ also gathered data on the ways in which food was prepared and cooked, as well as on the use of particular oils, margarine, and butter. To estimate the daily intake of energy and nutrients, dietary data from the FFQ were evaluated using the dietary analysis software Food Processor program (ESHA Food Processor SQL version 10.9.0.0), with additional information on the types of foods consumed in Jordan.14
Anthropometric Measurements
Heights and weights of the children, pre-adolescents, and adolescents were measured. According to the body mass index (BMI)-for-age Centers for Disease Control and Prevention (CDC) growth charts, underweight, overweight, and obese values were classified as follows: underweight percentile < 5, healthy weight ≥5 and <85, overweight ≥85 and<95, and obesity ≥ 95.15 Body weight was measured while the participants wore only light clothing and no shoes. In addition, the subjects’ height was measured while standing without wearing shoes, their shoulders relaxed, and their arms hanging freely (to the nearest 0.5 cm).
Biochemical Tests
A trained lab worker collected blood samples from each participant, drawing 5 mL of blood with a 3-mL syringe and a simple tube filled with gel. Triglyceride (TG), total cholesterol, high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL) levels were included in a group of blood tests called lipid profile parameters. Hemoglobin A1c (HbA1c) and fasting blood glucose (FBG) results were retrieved from the patients’ medical records.
Statistical Analysis
Data were analyzed using SPSS (version 22.0; IBM SPSS Statistics for Windows, IBM Corporation). Descriptive analyses were performed to investigate the frequencies of various variables. The Chi-square test was used to determine the statistical differences between categorical variables, Chi-square was used. Data are displayed as the mean ±SD. The normality of the distributions of the dietary consumption variables was evaluated using the Shapiro–Wilk test. The differences in daily nutrient intake for both groups were reported as median (33.3th-66.7th) and determined using a non-parametric one-sample T-test (Wilcoxon signed ranked test) for variables that were not normally distributed. Age, sex, daily insulin dose (units/kg/day), type of insulin, BMI, and calorie intake were adjusted for in all multivariate analyses.16 To simplify the calculation of odds ratios and provide a better understanding of the clinical implications of our findings, all macronutrients and micronutrients were divided into tertiles. P-value < 0.05 was reflected as statistically significant.16 Multinomial logistic regression analysis was used to evaluate the associations between macronutrient intake, micronutrient intake, and dyslipidemia. The model was evaluated using three goodness-of-fit chi-square statistics performed with dyslipidemia as the dependent variable in the presence of one or more abnormal lipid and lipoprotein levels. The threshold values for total cholesterol were ≥ 200 for total cholesterol, ≥ 100 for TG in patients aged–6-9 years old and ≥130 for patients aged–9-19 years old, 130 for LDL cholesterol, and < 35 for HDL cholesterol.17 The National Cholesterol Education Program and Expert Panel on Cholesterol Levels in Children provided values for the plasma lipid and lipoprotein levels. Non-HDL-C levels in Bogalusa were equal to the LDL cutoffs for the National Cholesterol Education Program Pediatric Panel.17 Normolipidemic and dyslipidemia groups were formed in the study sample. P values for trends were assessed using a linear logistic regression test.
Results
Table 1 presents descriptive, anthropometric, and biochemical information for the participants who were divided into two groups based on their lipid profiles. Participants (N= 90) were classified as dyslipidemic if there was at least one abnormal lipid profile variable (n= 33). Between the two dyslipidemic and normolipidemic groups, there was a significant difference in age groups (P=0.044). Additionally, statistically significant differences were observed in the incidence of disease (P=0.015), insulin type (P=0.041), morning insulin dose (P=0.007), and bedtime insulin dose (P=0.014). However, there were no statistically significant differences in weight (P=0.469), height (P= 0.326), and BMI (P=0.057). There were no significant differences in HbA1c and FBG levels between the dyslipidemia and normolipidemic groups (P=0.519 and P=0.474, respectively). The lipid profiles of diabetic children with dyslipidemia and those without dyslipidemia (mean SD and median) showed significantly higher levels of cholesterol (P=0.001), LDL (P<0.001), and TG (P<0.001). However, no statistical difference was detected in HDL levels (P=0.559).
 |
Table 1 Descriptive, Clinical, Anthropometric and Biochemical for Participants with and without Dyslipidemia |
The macronutrient intakes of the normolipidemic and dyslipidemic groups are shown in Table 2. The median values for both the groups were similar. For the normolipidemic group, the median daily energy intake was 2451.7 (2157.2–2667.3) Kcals, while for the dyslipidemic group, it was 2428.9 (2032.0–2781.0) Kcals. There were no noticeable differences between any of the macronutrients (P=0.877). There was no statistically significant difference (P=0.461) between the dyslipidemia and normolipidic groups; however, the dyslipidemia group had a greater median energy from fat intake (kcal). In contrast, the normolipidemic group had a greater median carbohydrate (g) value, although the difference was not statistically significant (P=0.196). When compared to the normolipidemic group, The median daily consumption of cholesterol (mg) was not significantly higher in the dyslipidemic group as compared to normolipidemic group (253.2 (155.5–327.6) vs.196.0 (131.9–252.0), P=0.214).
 |
Table 2 Daily Intake of Macronutrients of Normolipidemia and Dyslipidemia Participants with T1DM |
Regarding micronutrient intake of the normolipidemic and dyslipidemic groups, there were no significant differences between any of the medians in either group, except for vitamin B12 (Table 3). Table 3 shows that the dyslipidemic patients consumed significantly (P=0.046) more vitamin B12 than normolipidemic patients, with a median intake of 3.6(2.8–4.0) µg vs 2.7(2.1–3.5) µg, P= 0.046).
 |
Table 3 Daily Intake of Micronutrients of Normolipidemia and Dyslipidemia Participants with T1DM |
Table 4 and Table 5 provide the odds ratios and corresponding 95% confidence intervals for each nutrient after being divided into tertiles depending on whether the study participants had dyslipidemia. Age, sex, daily insulin dose (units/kg/day), type of insulin, height, weight, BMI, presence of disease, and calorie consumption were adjusted using multivariate analysis. No significant association was found between dyslipidemia and nutrient intake.
 |
Table 4 Association of Macronutrients Intake and Dyslipidemia Among Normolipidemia and Dyslipidemia Participants with T1DM |
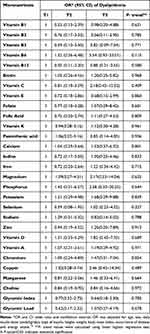 |
Table 5 Association of Micronutrients Intake and Dyslipidemia Among Normolipidemia and Dyslipidemia Participants with T1DM |
Discussion
Diabetes mellitus (DM) is an epidemic proportion.7 T1DM accounts for 5–10% of all DM cases of DM worldwide.7 The data indicate that T1DM has been augmented at an average rate of 3–4% per year in children and adolescents.7 Patients with T1DM have a 2–4 times greater risk of developing atherosclerosis earlier in life, leading to increased morbidity and mortality compared to people without DM.18 Moreover, cardiovascular events account for > 44% of the total mortality among these patients.19 Studies have reported serum lipid abnormalities in children with T1DM.18 An association between elevated HbA1c levels and serum lipid abnormalities.18,20
In this cross-sectional study, among Jordanian children and adolescents with T1DM, the prevalence of dyslipidemia was 37%. Several studies have indicated that the prevalence of dyslipidemia in children with T1DM varies between 29% and 66%.2,21–23 Our results are consistent with those of a study conducted among 202 children and adolescents with T1DM, in which 26.2% of the children had dyslipidemia.7 In addition, data from a study conducted in Turkey showed that the prevalence of dyslipidemia among adolescents with T1DM was 30.3%.24 The current data also agree with the findings of a case-control study that found that the prevalence of dyslipidemia was significantly higher among children and adolescents with T1DM (65%) than in the non-diabetic control group (28.2%).25
However, this is relatively lower than a retrospective study conducted among Egyptian children and adolescents with T1DM, which reported a prevalence of (70.47%).7 The wide range of dyslipidemia frequency among children with diabetes in various studies may be due to multiple genetic factors in different ethnic groups, local dietary habits, age ranges, and different reference ranges for dyslipidaemia.2,23
The current results showed that children with dyslipidemia had significantly higher levels of TGs, LDL-C, and total cholesterol (TC) than diabetic children with a normal lipid profile. This is in concordance with data from a previous study that found that among Egyptian diabetic children, hypertriglyceridemia was the predominant type of dyslipidemia and reported higher levels of HbA1c among untreated newly diagnosed children with T1DM than among diabetic children with good glycemic control.26,27 Also, have found that hypercholesterolemia was the most common type of dyslipidaemia and hypertriglyceridemia was the least common among diabetic patients.28 Data from a cross-sectional study conducted among patients with T1DM aged 2–18 years indicated that 67.3% had at least one abnormality in the serum lipid profile, and the predominant lipid profile abnormality was associated with elevated LDL levels (62% abnormality).7 These differences are usually related to different glycemic controls in various studies.28
According to data from the SEARCH for Diabetes in Youth (SEARCH) study, lipid abnormalities are more prevalent in children and adolescents with poor or suboptimal glycemic control.29 In addition, data from the SEARCH study reported that the total and LDL-C, TGs, non-HDL-C, and apolipoprotein B concentrations increased as HbA1c increased.29 Diabetic dyslipidemia is characterized by decreased HDL-C levels and increased LDL-C, and TGs.24 The American Heart Association has categorized children with TIDM as having the highest risk of cardiovascular disease and has recommended both lifestyle changes and pharmacological treatment for those with elevated LDL levels.30
The Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence (2014) recommends screening for fasting blood lipids when DM is stabilized in children over 10 years of 10 years.31,32 Moreover, if there is a family history of hypercholesterolemia or early cardiovascular diseases (CVDs) or if the family history is unknown, screening should be started at the age of 2 years.31,32 However, if normal lipid profiles have been obtained, screening should be repeated every 5 years.31,32
The current findings indicate that there were no significant differences in HbA1c and FBG levels between diabetic children with dyslipidemia and those with normolipidaemia. Several studies have indicated that poor glycemic control is associated with elevated serum lipid levels.22,33 It has been suggested that glycemic control is an important modifiable risk factor that is associated with the development of dyslipidemia as well as in the development of hypertriglyceridemia.33 It has been found that long-term glycemic management predicts the presence of atherosclerotic plaques on ultrasonography even in the absence of symptoms of coronary vascular disease.34 Intensive insulin therapy has also been reported to improve cardiovascular events in patients with childhood-onset DM.34 A few mechanisms have been hypothesized to show a direct effect of hyperglycemia on gene transcription of coagulation factors.35
Data have indicated that Insulin is a natural antagonist of the platelet hyperactivity.35 It enhances endothelial generation of nitric oxide (NO) and prostacyclin (PGI2), and sensitizes platelets to PGI.35 Therefore, long-term insulin deficiency in patients with T1DM, in combination with poor glycemic control, dyslipidemia, and dysfunctional coagulation cascade, may provide a significant risk for developing dyslipidemia.35 Additionally, the current data indicated that there were statistical differences in the incidence of disease (P-value<0.05) between children with dyslipidemia and normolipidemic diabetes. The current data are inconsistent with several previous studies that have shown that there were no significant differences in the duration of DM between the dyslipidemic and normolipidemic groups.22,36 Dyslipidemia was observed among children and adolescents with T1DM regardless of the duration of diabetes.22,36
The current data agree with the results reported by Moayeri and Oloomi (2006), who found that lipid levels were positively correlated with DM duration of DM.37 Several studies have revealed that C-reactive protein is elevated during the first year of T1DM diagnosis, and both interleukin 6 and fibrinogen levels are elevated in individuals with an average disease duration of 2 years.38–40 The current results showed that there were no significant differences in macronutrient intake between diabetic children with dyslipidemia and those with normolipidemia. However, the dyslipidemia group had a greater median energy from fat intake. It has been demonstrated that the adherence to macronutrient recommendations was the most important aspect among youth with T1DM.41 Several studies have revealed that children and adolescents with T1DM consumed more fat and saturated fat than age-based recommendations, and more than healthy controls.41–43
Moreover, Patton (2011) found that the total percentage of energy from fat in children and adolescents ranged from 31% to 47%, which was higher than the Healthy People 2010 recommendation of less than 30%.41 In addition, the mean percentage of energy from saturated fat ranged from 11% to 15%, which was higher than the American Diabetes Association recommendation of less than 7%.44 Youth with T1DM consumed significantly less than the recommended number of servings of both fruits and vegetables per day.45 However, young children with T1DM reported a higher proportion of energy from vegetables (10% vs 6%) compared to controls and a similar proportion of daily energy from fruits (10% vs 12%) compared to controls.42
Low fiber consumption has been reported to significantly increase TC, TGs, and VLDL concentrations.46 This could be explained by decreased cholesterol absorption, decreased hepatic cholesterol synthesis, and decreased levels of circulating cholesterol due to increased cholesterol use, which is driven by intestinal bile acid sequestration by fiber.46 Moreover, it has been demonstrated that the Mediterranean-style diet has been improve dyslipidemia, in particular LDL-C, and reduce the risk of CVDs.47
The major strength of this study is the use of an ethnically validated FFQ. In addition, this study is one of the few Jordanian studies to assess dyslipidemia among children and adolescents with TIDM. Furthermore, the inclusion of data on physical activity, body weight, and dietary intake, all of which are known to influence lipid levels, is also a strength of this study. The current study had several limitations. First, it was limited by the lack of assessment of various apolipoprotein levels and carotid artery intima-media thickness, which are both significant predictors of CVD risk. Second, because of the cross-sectional nature of the study, repeated measurements of fasting lipid profiles were not performed over time among diabetic children and adolescents. Additionally, even though we have adjusted the OR for many confounding factors, a residual effect may still persist. Finally, the study sample size was small compared with other studies from countries with higher populations. Therefore, studies with larger sample sizes are required to shed more light on this topic.
In conclusion, this study demonstrated a relatively high prevalence of dyslipidemia in the study children and adolescents with T1DM. High TGs, LDL-C, and TC levels were the most frequent abnormalities observed in the dyslipidemic group. The study did not show any association between macro and micronutrients and dyslipidemia in the study participants. As dyslipidemia is a modifiable risk factor for CVD among children and adolescents with T1DM, longitudinal studies are needed to evaluate the effects of early diagnosis and treatment of dyslipidemia on the progression of complications. Moreover, annual screening for dyslipidemia in children and adolescents with TIDM is recommended. The current study supports the need for nutritional guidance for the treatment of dyslipidemia in this population.
Ethical Approval
The study protocol received approval from the Institutional Review Board (IRB) committee of King Hussein Medical Center (Queen Rania Pediatric Hospital), aligning with the ethical standards outlined in the 1975 Declaration of Helsinki. The IRB number for the study was 1/2019/2863.
Acknowledgments
The authors would like to express their gratitude to the Deanship of Scientific Research at The University of Jordan for funding the research projects. Special thanks to Eng. Rana Al-Z’ubi from King Hussein Medical Center for her efforts in participant recruitment. Additionally, we extend our thanks to the children, adolescents, and their parents for agreeing to be part of this study. Open Access funding provided by the Qatar National Library.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Gujral J, Gupta J. Pediatric Dyslipidemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
2. Homma TK, Endo CM, Saruhashi T, et al. Dyslipidemia in young patients with type 1 diabetes mellitus. Arch Endocrinol Metab. 2015;59(3):215–219. doi:10.1590/2359-3997000000040
3. Pappan N, Rehman A. Dyslipidemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
4. Yanai H, Yoshida H. Secondary dyslipidemia: its treatments and association with atherosclerosis. Glob Health Med. 2021;3(1):15–23. doi:10.35772/ghm.2020.01078
5. Mainieri F, La Bella S, Chiarelli F. Hyperlipidemia and cardiovascular risk in children and adolescents. Biomedicines. 2023;11(3):809. doi:10.3390/biomedicines11030809
6. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–2738. doi:10.1161/circulationaha.106.179568
7. Soliman H, Ibrahim A. Prevalence and pattern of dyslipidemia in an Egyptian children and adolescents with type 1 diabetes. Egypt Ped Assoc Gazet. 2021;69(1):21. doi:10.1186/s43054-021-00067-x
8. Lee J, Hoang T, Lee S, et al. Association between dietary patterns and dyslipidemia in Korean Women. Front Nutr. 2021;8:756257. doi:10.3389/fnut.2021.756257
9. Nicklas TA, Dwyer J, Feldman HA, et al. Serum cholesterol levels in children are associated with dietary fat and fatty acid intake. J Am Diet Assoc. 2002;102(4):511–517. doi:10.1016/s0002-8223(02)90117-3
10. Weidman WH, Elveback LR, Nelson RA, et al. Nutrient intake and serum cholesterol level in normal children 6 to 16 years of age. Pediatrics. 1978;61(3):354–359.
11. Sirajudeen MS, Waly M, Manzar MD, et al. Physical activity questionnaire for older children (PAQ-C): Arabic translation, cross-cultural adaptation, and psychometric validation in school-aged children in Saudi Arabia. PeerJ. 2022;10:e13237. doi:10.7717/peerj.13237
12. Kowalski KC, Crocker PRE, Donen RM. The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual. Saskatoon: College of Kinesiology University of Saskatchewan; 2004:11–15.
13. Tayyem RF, Albataineh SR, Allehdan S, et al. Development and validation of a food frequency questionnaire for assessing nutrient intake during childhood in Jordan. Nutr Hosp. 2020;37(6):1095–1106. doi:10.20960/nh.03079
14. Takruri HA, Tayyem R, Al-Dabbas M. Composition of Local Jordanian Food Dishes. Amman, Jordan: Dar Zuhdi; 2020.
15. Lee RND. Nutritional Assessment. New York, NY, USA: McGraw-Hill Companies, Inc.; 2013.
16. Maffeis C, Morandi A, Ventura E, et al. Diet, physical, and biochemical characteristics of children and adolescents with type 1 diabetes: relationship between dietary fat and glucose control. Pediat Diabet. 2012;13(2):137–146. doi:10.1111/j.1399-5448.2011.00781.x
17. Kwiterovich PO. Recognition and management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab. 2008;93(11):4200–4209. doi:10.1210/jc.2008-1270
18. Shamir R, Kassis H, Kaplan M, et al. Glycemic control in adolescents with type 1 diabetes mellitus improves lipid serum levels and oxidative stress. Pediatr Diabetes. 2008;9(2):104–109. doi:10.1111/j.1399-5448.2007.00313.x
19. Lemkes BA, Hermanides J, DeVries JH, et al. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8(8):1663–1669. doi:10.1111/j.1538-7836.2010.03910.x
20. Zabeen B, Balsa AM, Islam N, et al. Lipid profile in relation to glycemic control in type 1 diabetes children and adolescents in Bangladesh. Indian J Endocrinol Metab. 2018;22(1):89. doi:10.4103/ijem.IJEM_217_17
21. Wiltshire EJ, Hirte C, Couper JJ. Dietary fats do not contribute to hyperlipidemia in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26(5):1356–1361. doi:10.2337/diacare.26.5.1356
22. Guy J, Ogden L, Wadwa RP, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32(3):416–420. doi:10.2337/dc08-1775
23. Kim S-H, Jung I-A, Jeon YJ, et al. Serum lipid profiles and glycemic control in adolescents and young adults with type 1 diabetes mellitus. Anna Ped Endocrinol Metab. 2014;19(4):191. doi:10.6065/apem.2014.19.4.191
24. Bulut T, Demirel F, Metin A. The prevalence of dyslipidemia and associated factors in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2017;30(2):181–187. doi:10.1515/jpem-2016-0111
25. Mona HM, Sahar SA, Hend SM, et al. Dyslipidemia in type 1 diabetes mellitus: relation to diabetes duration, glycemic control, body habitus, dietary intake and other epidemiological risk factors. Egypt Ped Assoc Gazet. 2015;63(2):63–68. doi:10.1016/j.epag.2015.03.001
26. Herman WH, Aubert R, Engelgau M, et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complications. Diabetic Med. 1998;15(12):1045–1051. doi:10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L
27. Kantoosh MM, Naiem AM, El-Sayad M, et al. Dyslipidemia and lipid peroxidation in type 1 diabetic children with good glycemic control: response to antioxidant therapy. Alex J Pediatr. 2002;16:357–364.
28. Patiakas S, Kiriakopoulos N, Gavala C, et al. The lipid profile of patients with diabetes mellitus in Paionia county. Diabetol Stoffw. 2007;2(04):A35. doi:10.1055/s-2007-984781
29. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–3344. doi:10.2337/dc14-0574
30. McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and obesity in youth committee, council of cardiovascular disease in the young, with the council on cardiovascular Nursing. Circulation. 2007;115(14):1948–1967. doi:10.1161/CIRCULATIONAHA.107.181946
31. Donaghue KC, Wadwa RP, Dimeglio LA, et al. Microvascular and macrovascular complications in children and adolescents. Pediat Diabet. 2014;15(S20):257–269. doi:10.1111/pedi.12180
32. Topouchian J, Agnoletti D, Blacher J, et al. Validation of four devices: omron M6 Comfort, Omron HEM-7420, Withings BP-800, and Polygreen KP-7670 for home blood pressure measurement according to the European society of hypertension international protocol. Vascul Health Risk Manag. 2014;10:33–44. doi:10.2147/VHRM.S53968
33. Teles SAS, Fornés NS. Relationship between anthropometric and biochemical profiles in children and adolescents with type 1 diabetes. Rev Paulista Pediatria. 2012;30:65–71. doi:10.1590/S0103-05822012000100010
34. Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi:10.2337/dc13-2112
35. Vinik AI, Erbas T, Park TS, et al. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485. doi:10.2337/diacare.24.8.1476
36. Kanagalakshmi K, Sultana A. Preliminary study of lipid profiles in pediatric population and youth population with type 1 diabetes. Int J Pharm Biol Sci. 2012;3:828–832.
37. Moayeri H, Oloomi Z. Prevalence of dyslipidemia in children and adolescents with diabetes mellitus type I. Iran J Pediatr. 2006;16(2):171–176.
38. Hayaishi-Okano R, Yamasaki Y, Katakami N, et al. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care. 2002;25(8):1432–1438. doi:10.2337/diacare.25.8.1432
39. Schölin A, Siegbahn A, Lind L, et al. CRP and IL‐6 concentrations are associated with poor glycemic control despite preserved β‐cell function during the first year after diagnosis of type 1 diabetes. Diabetes/Metab Res Rev. 2004;20(3):205–210. doi:10.1002/dmrr.427
40. Abed E, LaBarbera B, Dvorak J, et al. Prevalence of dyslipidemia and factors affecting dyslipidemia in young adults with type 1 diabetes: evaluation of statin prescribing. J Pediatr Endocrinol Metab. 2019;32(4):327–334. doi:10.1515/jpem-2018-0383
41. Patton SR. Adherence to diet in youth with type 1 diabetes. J Am Diet Assoc. 2011;111(4):550–555. doi:10.1016/j.jada.2011.01.016
42. Virtanen SM, Ylönen K, Räsänen L, et al. Two year prospective dietary survey of newly diagnosed children with diabetes aged less than 6 years. Arch Dis Child. 2000;82(1):21–26. doi:10.1136/adc.82.1.21
43. Overby NC, Margeirsdottir HD, Brunborg C, et al. The influence of dietary intake and meal pattern on blood glucose control in children and adolescents using intensive insulin treatment. Diabetologia. 2007;50(10):2044–2051. doi:10.1007/s00125-007-0775-0
44. Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78.
45. Mayer-Davis EJ, Nichols M, Liese AD, et al. Dietary intake among youth with diabetes: the SEARCH for diabetes in youth study. J Am Diet Assoc. 2006;106(5):689–697. doi:10.1016/j.jada.2006.02.002
46. Fernandez M-L. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12(1):35–40. doi:10.1097/00041433-200102000-00007
47. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi:10.1001/jama.292.12.1440
 © 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
