Back to Journals » Drug Design, Development and Therapy » Volume 9
Antitumoral materials with regenerative function obtained using a layer-by-layer technique
Authors Ficai D, Sonmez M, Albu MG, Mihaiescu DE, Ficai A, Bleotu C
Received 20 February 2014
Accepted for publication 3 December 2014
Published 2 March 2015 Volume 2015:9 Pages 1269—1279
DOI https://doi.org/10.2147/DDDT.S62805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Shu-Feng Zhou
Denisa Ficai,1 Maria Sonmez,1,2 Madalina Georgiana Albu,2 Dan Eduard Mihaiescu,1 Anton Ficai,1 Coralia Bleotu3
1Faculty of Applied Chemistry and Material Science, Politehnica University of Bucharest, 2Leather and Footwear Research Institute, National Research and Development Institute for Textiles and Leather, 3Stefan S Nicolau Institute of Virology, Romanian Academy, Bucharest, Romania
Abstract: A layer-by layer technique was successfully used to obtain collagen/hydroxyapatite-magnetite-cisplatin (COLL/HAn-Fe3O4-CisPt, n=1–7) composite materials with a variable content of hydroxyapatite intended for use in the treatment of bone cancer. The main advantages of this system are the possibility of controlling the rate of delivery of cytostatic agents, the presence of collagen and hydroxyapatite to ensure more rapid healing of the injured bone tissue, and the potential for magnetite to be a passive antitumoral component that can be activated when an appropriate external electromagnetic field is applied. In vitro cytotoxicity assays performed on the COLL/HAn-Fe3O4-CisPt materials obtained using a layer-by layer method confirmed their antitumoral activity. Samples with a higher content of hydroxyapatite had more antitumoral activity because of their better absorption of cisplatin and consequently a higher amount of cisplatin being present in the matrices.
Keywords: multifunctional materials, antitumoral activity, scaffold, bone grafts
Introduction
Cancer is one of the leading causes of death in the 21st century.1 Osteosarcoma is a frequent type of bone cancer that occurs particularly in childhood and adolescence.2 The evolution of chemotherapy in osteosarcoma has taken four major turns: surgery only (before 1970); surgery + adjuvant chemotherapy (1970–1990, include cisplatin-based and Pt-based cytostatic agents); surgery + neoadjuvant chemotherapy (1990–2000);3 and surgery + neoadjuvant chemotherapy + unconventional methods (2000+, including phototherapy,4 hyperthermia,5 nanoparticles used for both imaging or cancer treatment,6 local radiotherapy,7,8 immunotherapy9).
Researchers are currently focused on identifying new drugs or improving administration of existing ones, reflecting a general trend in the treatment of cancer. Some important improvements were achieved with the development of drug delivery systems. Development of new drugs is quite difficult, and involves high costs, so better administration seems to be a more reasonable way to improve the quality of the medical care available.10,11 In general, improvements related to use of drug delivery systems are because of the following: localized/targeted delivery, long-term delivery, and control of the curative ability of the active component at the site of interest. Drug delivery can be controlled by four main strategies, ie, physical, chemical, biological, and mechanical.12
Cisplatin was discovered in 1844,13 and in clinical trials as far back as 197014 was being used to treat a number of different types of cancer, usually as part of combination therapy for cervical carcinoma, lymphoma, osteosarcoma, and melanoma, and testicular, ovarian, head and neck, bladder, and non-small cell and small cell lung cancers.15 The mechanism of action of cisplatin involves an interaction with the N7 guanine from DNA, leading to DNA damage/breaks, and depending on the dose of cisplatin and the state of the cell, cisplatin may induce cell death via a defective apoptotic program or even by necrosis.16–18 Intermediate, cisplatin is hydrolyzed, both chlorine atoms being substituted with water inside the cell where the chlorine concentration is about 3–20 mM. This reaction does not occur in the bloodstream because of its high chlorine concentration (about 100 mM). Comparing with the trans isomer, in the case of cisplatin the steric arrangement of the two chlorines allows a coordination reaction via formation of a loop.17,19 Cisplatin is also an active component in a number of combination formulations such as hyaluronic acid-gelatin-cisplatin,20 poly(lactic-co-glycolic acid)-cisplatin,21 collagen/hydroxyapatite-cisplatin,22 Fe3O4@K2Ti4O9-cisplatin,23 SiO2-cisplatin,24 and hydroxyapatite-cisplatin.25
Magnetite has certain specific properties that are potentially useful in the treatment of cancer. These include an ability to accumulate in the target tissue/organ due to its magnetic properties, an ability to induce hyperthermia due to hysteresis loss and an enhanced ability to deliver antitumoral agents when an alternating electromagnetic field is applied. Hyperthermia-enhanced antitumoral efficacy of a number of cytostatic agents has been studied in vitro and in vivo,26–28 and can be explained on the basis of delivery of an increased amount of cytostatic drug when an electromagnetic field is applied. The increasing temperature produced by hyperthermia leads to increasing Brownian motion which means increasing diffusion/delivery rate.
The aim of this work was to obtain a multifunctional collagen/hydroxyapatite-magnetite-cisplatin (COLL/HA-Fe3O4-CisPt) composite material with dual antitumoral activity ensured by release of cisplatin and hyperthermia. A layer-by-layer (LbL) method was used to tune the antitumoral activity of these materials by controlling the rate of release of the chemotherapeutic agent. These materials, when exposed to an appropriate electromagnetic field, can induce local hyperthermia; the main advantages of using hyperthermia when compared with classical chemotherapy are reduced toxicity, longer activity, and use of less cisplatin without affecting its activity. Based on the actual protocol, tumoral tissue resection is recommended. In these cases, the multifunctional systems can be loaded into the defects. The cytostatics are delivered and after the delivery, the remanent COLL/HAn(-Fe3O4) act as a support for bone regeneration/healing.
Materials and methods
Collagen (300,000 Da) gel was obtained from the Collagen Department at the Leather and Footwear Research Institute, starting with calf hides and chemical and enzymatic extraction.29 Beginning with collagen gel, collagen matrices were obtained by crosslinking with glutaraldehyde and freeze-drying to preserve the porous microstructure.30 These collagen matrices are widely known for having a porous structure (with open porosity of over 95%).31
The antitumoral and control samples were synthesized as follows. In first step, magnetite was deposited onto the desired collagen matrices (40 collagen matrices ~1×1 cm in size were used, each weighing ~0.0250 g) by coprecipitation of FeCl3 and FeCl2 in a molar ratio of 2:1. NaOH 0.1 M was then added dropwise until a black precipitate was obtained. In this step, COLL/Fe3O4 matrices containing ~8% Fe3O4 were obtained (reported to the initial mass of collagen, ~0.0250 g). Three of these COLL/Fe3O4 matrices were used as control samples, while the others were used for COLL/HA-Fe3O4 samples. Next, COLL/Fe3O4 matrices were mineralized using the LbL method, gently pressed to partially eliminate water before absorption of cisplatin. In brief, mineralization occurs by successive immersion of the collagen matrices into Ca(OH)2 suspension 3.4360 g/L and NaH2PO4 solution 4.4672 g/L, as described in our previous papers.32,33 Two samples from COLL/HAn-Fe3O4 were obtained, with one sample loaded with 1mL of 10 μM cisplatin solution while the second with 2mL 10 μM cisplatin solution. Cisplatin was added to each matrix to obtain antitumoral samples that would release cisplatin 5 μM or 10 μM for in vitro tests. These two concentrations were selected on the basis of our previous data obtained in a human G292 osteosarcoma cell line where we determined the IC50 for pure cisplatin to be ~6.1 μM.34 A volume and concentration of cisplatin was chosen that would ensure complete adsorption of cisplatin onto the matrix and homogenous distribution of the drug. Only antitumoral samples with one, three, five, and seven layers (LbL1, LbL3, LbL5, and LbL7, respectively) were obtained and tested.
The obtained materials were investigated by X-ray diffraction and scanning electron microscopy (SEM) as well as by determining the release profile of cisplatin and its influence on HCT8 tumoral cells. X-ray diffraction analysis was performed using an XRD 6000 diffractometer (Shimadzu, Tokyo, Japan) at room temperature. In all cases, Cu Kα radiation from a Cu X-ray tube was used. The samples were scanned in the Bragg angle (2θ range 10°–70°). SEM was performed using an S2600N electron microscope (Hitachi, Tokyo, Japan) on samples covered with a layer of silver.
A study of cisplatin delivery was performed using a high-performance liquid chromatography system (Agilent 1100, Agilent Technologies, Santa Barbara, CA, USA) under online conditions (ie, direct injection in the ultraviolet detector, with no chromatographic column inserted into the system) at 280 nm in 0.9% saline solution. For this reason, each COLL/HA-Fe3O4-CisPt sample was immersed in 50 mL of 0.9% saline solution and absorbance was recorded over 7 hours of release. The data were plotted against time to obtain the curve for release of cisplatin from the multifunctional COLL/HA-Fe3O4-CisPt composite materials obtained by the LbL technique (LbLn-Fe3O4-CisPt, where n is the number of layers).
Cell viability
The eukaryotic cell culture used in our assays was represented by HeLa (ATCC® CCL-2™, American Tissue Culture Collection, Manassas, VA, USA) that was maintained as an adherent culture in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich) at 37°C and 5% CO2 in a humid atmosphere. Each sample obtained by the LbL technique (~0.5 cm2 LbLn-Fe3O4-CisPt, ~7 mm thickness) was injected with 2×105 cells/mL. After 24 hours, the effects of the labeled cisplatin were evaluated using a fluorescein diacetate and propidium iodide double-staining protocol.
Cell cycle analysis
First, 2×105 cells were seeded into each well of a 24-well plate. After cell recovery, LbL1-Fe3O4-CisPt, LbL3-Fe3O4-CisPt, LbL5-Fe3O4-CisPt, and LbL7-Fe3O4-CisPt were added to the medium. The cells were harvested after 24 hours and washed in a cold solution of phosphate-buffered saline (pH 7.5), then fixed in cold ethanol (70%) and stored at −20°C overnight. The samples were then centrifuged, washed with phosphate-buffered saline, and resuspended in 100 μL of phosphate-buffered saline, treated with RNase A (1 mg/mL) for 15 minutes at 37°C, labeled with propidium iodide (100 μg/mL) at 37°C, and incubated at 4°C for 30 minutes prior to measurement. The DNA content of the cells was quantified on an EPICS XL flow cytometer (Beckman Coulter, Brea, CA, USA) and analyzed using FlowJo version 8.8.6 software (Ashland, OR, USA).
In our experiments, we used HeLa cells, a tumor cell line derived from cervical cancer, because cisplatin is the drug of choice for treatment of cervical cancer and in certain other malignancies such as breast and bone cancers. Most of the studies regarding the mechanisms by which cisplatin induces its cytotoxic effects have been done using this cell line.35–37 Thus, the experimental model we used was appropriate to study the dynamics of cisplatin release from a substratum in culture medium and could be successfully used to select appropriate drug release systems. Both the in vitro study and the release study were performed in triplicate, and the results presented are the average of these data.
Results and discussion
X-ray diffraction
The X-ray diffraction patterns of the samples obtained by the LbL method were recorded, and the main peaks identified for magnetite and hydroxyapatite are shown in Figure 1. Even if cisplatin gives diffraction peaks, its concentration and low crystallinity make it impossible to clearly visualize the diffraction peaks of cisplatin, regardless its content (even in the case of COLL/Fe3O4-CisPt, which contained the highest amount of cisplatin Fe3O4:CisPt =1:1 [wt/wt]).
The relative intensity of the main peaks of hydroxyapatite (HA [121]) and magnetite (Fe3O4[311]) versus the number of layers shows a quasilinear region between one and seven layers, the intensity of HA [121] increasing with the increase in number of layers (Figure 2). This evolution can be attributed to the very good linearity of the deposition determined by gravimetry and/or thermogravimetry, which we proved in past research.32,33
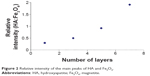  | Figure 2 Relative intensity of the main peaks of HA and Fe3O4. |
Scanning electron microscopy
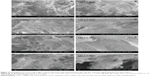
Morphological evaluation of COLL/Fe3O4-CisPt and COLL/HA-Fe3O4-CisPt was done because this parameter is essential for both drug delivery (especial for controlling the delivery rate and degree of recovery) and osseointegration. Regardless of the degree of mineralization of the samples, the presence of fibrillar collagen can be highlighted. The morphological variation in the samples appears to be due to the varying amounts of hydroxyapatite deposited during the LbL process. Due to the strong tendency of magnetite to agglomerate, SEM images were recorded for the COLL/Fe3O4-CisPt control sample (LbL0). The porosity of these materials decreased from ~98% for the pure collagen matrix down to ~75% for the most mineralized COLL/HA matrix (LbL7) obtained based on the Arthur method, ie, absorption of xylene.38 In backscattering mode (Figure 3A–C), a homogenous distribution of the magnetite particles is indicated by the uniform distribution of the Fe atoms throughout the matrix, with the same results being observed in the SEM images recorded in energy-dispersive X-ray mode (Figure 4). Due to the low content of cisplatin, the Pt could not be identified, so the contrast is attributed solely to the presence of magnetite (Fe).
The COLL/HA-Fe3O4-CisPt samples obtained by LbL showed an increasing level of mineral phase as the number of layers increased. This can be seen easily in the SEM images recorded at 2,500×. Representative SEM images of the samples obtained by LbL are shown in Figure 5, and are in good agreement with our previous studies.32,33
The distribution and content of the main elements (Ca, P, Fe, and Ag) was also analyzed by energy-dispersive X-ray spectroscopy (Figure 6). The constituent elements of magnetite (Fe) and hydroxyapatite (Ca, P) can be identified (as well as the Ag that was used for preparation of the sample) while Pt (from cisplatin) cannot be identified because of the low level of cisplatin.
In mapping mode, selecting Ca, P, Fe, and Ag it can be seen as a very homogenous material, with no agglomeration of magnetite (Figure 4).
Infrared spectroscopy
Infrared spectroscopy was used to characterize the antitumoral samples obtained by the LbL technique as well as the most important intermediary products. As previously established, LbL is a powerful technique for mineralization of collagen, the amount of hydroxyapatite being proportional to the number of deposited layers. Cisplatin is also identifiable at ~1,300 cm−1, this absorption band being not superimposed on other bands assignable to hydroxyapatite, collagen, or magnetite. At ~2,922 cm−1, 2,850 cm−1, and ~3,285 cm−1 the intense absorption bands of cisplatin are overlapped over the collagen bands and cannot be clearly identified.32,34
Support-cisplatin interaction
The physical and chemical interactions of cisplatin, especially with hydrogen bonds can appear between amino groups of cisplatin and any OH, NH, NH2, or COOH groups from collagen, hydroxyapatite, or magnetite. The hydrogen bonds are usually analyzed by infrared spectroscopy, the characteristic region being 3,000 cm−1 to 3,600 cm−1. It can be observed that this region is strongly dependent on composition. To highlight this dependence, the Fourier transform infrared spectra of the samples containing collagen and cisplatin (LbL0 + cisplatin); collagen, magnetite, and cisplatin (LbL0 + Fe3O4 + cisplatin); collagen, hydroxyapatite, magnetite, and cisplatin (LbL1 + Fe3O4 + cisplatin); and pure collagen (LbL0) were overlapped (Figure 7). Based on this analysis, it can be observed that the number of peaks (or shoulders), their intensity and position are modified, the overall shape and intensity of this large band being assigned to the formation of hydrogen bonds. It can be concluded that the hydrogen bond region is continuously changing with the addition of different components, with the changes between the pure collagen matrix and collagen + cisplatin being very important. Analyzing the Fourier transform infrared spectra of the antitumoral samples (Figure 8), it can be concluded that the increasing amount of hydroxyapatite leads to a stronger change in this region, practically the band from ~3,285 cm−1 being totally included in the broad band from 3,000 cm−1 to 3,600 cm−1, most probably due to the increasing content of hydroxyapatite as well as the stronger interactions between cisplatin and hydroxyapatite. These hydrogen bonds are also responsible for the low level of recovery of the cisplatin, and can explain why the antitumoral activity is not altered when cisplatin is adsorbed on the support, the mechanism of antitumoral activity involving the substitution of the two chlorides from cisplatin with guanine (from DNA).39
  | Figure 7 Fourier transform infrared spectra of the samples. |
  | Figure 8 Fourier transform infrared spectra of the different samples containing collagen, hydroxyapatite, magnetite, and cisplatin. |
Cisplatin release study
Cisplatin release is dependent on processing as well as on the external electromagnetic field applied.28 Previously we demonstrated that the LbL technique can be used to obtain composite collagen/hydroxyapatite materials with an increasing content of hydroxyapatite, the content of hydroxyapatite being directly proportional to the number of successive layers.32,33 The release curves for cisplatin from the multifunctional materials are presented in Figure 9, cisplatin release being inversely proportional to the number of deposited layers. It can be seen that the delivery profiles are strongly dependent on the number of layers, especially for the samples with a low content of hydroxyapatite (ie, a low number of layers), while the samples obtained by LbL5 and LBL7 show almost the same release. This can be explained as follows: as the number of deposited layers increases, the porosity of the materials decreases slightly and consequently the delivery rate decreases slightly; the increase in hydroxyapatite content involves modification of the support-cisplatin interactions so, based on the release profile, the interaction between hydroxyapatite and cisplatin is much stronger than that between collagen and cisplatin.
The recovery of cisplatin following 8 hours of release is about 86.5% for the material obtained by LbL1, 70% for that obtained by LbL3, and ~65% for that obtained by LbL5 and LbL7. Cisplatin release would be expected to be slower when implanted in bony tissue than in saline solution due to the lower humidity, different ionic strength, higher viscosity, and especially slower diffusion.
Cytotoxic effects of the composite materials on tumor cells
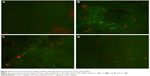
The cisplatin delivered from the four analyzed samples (COLL/HAn-Fe3O4-CisPt obtained by LbL1, LbL3, LbL5, and LbL7) over 24 hours induced high mortality of HeLa cells injected into sponges, as quantified by the fluorescein diacetate and propidium iodide double-staining procedure (see Figure 10). In addition to the previous analysis, cell cycle analysis (Figure 11) showed a dramatic decrease in G2 phase as well as the appearance of a subG0 peak associated with apoptotic cell death. DNA replication (S phase) was also altered, this effect being easily explained by the mechanism of action of cisplatin.
Conclusion
The LbL technique was used to obtain composite COLL/HAn-Fe3O4-CisPt materials with a regenerative and antitumoral role that could be effective for controlling the rate of delivery of cisplatin. Cisplatin release was controlled by the material/fabrication of the support as well as by the external electromagnetic field applied, with the temperature increase being favorable for more rapid delivery. As a direct result of this control, the COLL/HA-Fe3O4-CisPt materials obtained by the LbL technique showed different degrees of cytotoxicity in a tumor cell line. The cytotoxicity was dependent on the cisplatin content; the lowest antitumoral activity was obtained at 5 μM cisplatin and increased for samples releasing 10 μM cisplatin. Further, cytotoxicity depended on the number of layers of the composite materials. Further work will be done in vitro in primary bone cancers and on metastasized cancers in vivo.
Acknowledgment
The authors acknowledge the financial support of the European Social Fund through the POSDRU/89/1.5/S/54785 project for Postdoctoral Program for Advanced Research in the Field of Nanomaterials.
Disclosure
The authors report no conflicts of interest in this work.
References
Hiatt RA, Rimer BK. A new strategy for cancer control research. Cancer Epidemiol Biomarkers Prev. 1999;8:957–964. | ||
Longhi A, Errani C, Paolis MD, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. | ||
Somjee S, Pai SK, Advani SH. Evolution of chemotherapy in osteosarcoma. Indian J Med Paediatr Oncol. 2001;22:24–27. | ||
Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–13554. | ||
Andronescu E, Ficai M, Voicu G, Ficai D, Maganu M, Ficai A. Synthesis and characterization of collagen/hydroxyapatite:magnetite composite material for bone cancer treatment. J Mater Sci Mater Med. 2010;21:2237–2242. | ||
McNeil SE. Nanoparticle therapeutics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:264–271. | ||
Mitra A, Coleman T, Borgman M, Nan A, Ghandehari H, Line Braach-Maksvytis V. Polymeric conjugates of mono- and bi-cyclic alpha(V)beta(3) binding peptides for tumor targeting. J Control Release. 2006;114:175–183. | ||
Qu C, Song E, Li Y, et al. Pre-clinical study of 213Bi labeled PAI2 for the control of micrometastatic pancreatic cancer. Clin Exp Metastasis. 2005;22:575–586. | ||
Hofmeister V, Vetter C, Schrama D, Bröcker E-B, Becker JC. Tumor stroma-associated antigens for anti-cancer immunotherapy. Cancer Immunol Immunother. 2006;55:481–494. | ||
Collier R. Drug development cost estimates hard to swallow. Can Med Assoc J. 2009;180:279–280. | ||
Dutta RC. Drug carriers in pharmaceutical design: promises and progress. Curr Pharm Des. 2007;13(7):761–769. | ||
Robinson DH, Mauger JW. Drug delivery systems. Am J Hosp Pharm. 1991;48:S14–S23. | ||
Peyrone M. Ueber die Einwirkung des Ammoniaks auf Platinchlorür [The influence of ammonia on platinum chloride]. Justus Liebigs Annalen der Chemie. 1844;51:1–29. German. | ||
Jones EG. Cisplatin. Available from: http://www.cisplatin.org/. Accessed April 8, 2012. | ||
Natile G, Colucci M. Current status of trans-platinum compounds in cancer therapy. Coord Chem Rev. 2001;216–217:383–410. | ||
Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. | ||
Hou X-M, Zhang X-H, Wei K-J, et al. Cisplatin induces loop structures and condensation of single DNA molecules. Nucleic Acids Res. 2009;37:1400–1410. | ||
Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59:657–663. | ||
Smith A. Cisplatin as an anticancer drug. Cisplatin: The Invention of an Anticancer Drug. Hamden CT, USA: Quinnipiac University, 2005. Available from: http://www.chemcases.com/cisplat/index.htm. Accessed December 17, 2014. | ||
Chen JP, Leu YL, Fang CL, Chen CH, Fang JY. Thermosensitive hydrogels composed of hyaluronic acid and gelatin as carriers for the intravesical administration of cisplatin. J Pharm Sci. 2011;100:655–666. | ||
Lee YS, Lowe JP, Gilby E, Perera S, Rigby SP. The initial release of cisplatin from poly(lactide-co-glycolide) microspheres. Int J Pharm. 2010;383:244–254. | ||
Andronescu E, Ficai A, Albu MG, et al. Collagen-hydroxyapatite/cisplatin drug delivery systems for locoregional treatment of bone cancer. Technol Cancer Res Treat. 2013;12:275–284. | ||
Kikkawa S, Takagi S, Tamura H. Preparation of titanate coated magnetite powder for cisplatin delivery. J Ceram Soc Jpn. 2008;116:380–383. | ||
Czarnobaj K, Lukasiak J. In vitro release of cisplatin from sol-gel processed organically modified silica xerogels. J Mater Sci Mater Med. 2007;18:2041–2044. | ||
Iafisco M, Palazzo B, Marchetti M, et al. Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. J Mater Chem. 2009;19:8385–8392. | ||
Oyama T, Kawamura M, Abiko T, et al. Hyperthermia-enhanced tumor accumulation and antitumor efficacy of a doxorubicin-conjugate with a novel macromolecular carrier system in mice with non-small cell lung cancer. Oncol Rep. 2007;17:653–659. | ||
Ito A, Fujioka M, Yoshida T, et al. 4-S-Cysteaminylphenol-loaded magnetite cationic liposomes for combination therapy of hyperthermia with chemotherapy against malignant melanoma. Cancer Sci. 2007;98:424–430. | ||
Ficai A, Andronescu E, Ghitulica DC, Ficai D, Albu MG. Inventors; Univ Politehnica Din Bucuresti, assignee. Process for preparing composite multi-purpose materials with possible applicability in the treatment of bone cancer. US Patent RO127725.A2. November 24, 2010. | ||
Ficai A, Andronescu E, Voicu G, et al. Self assembled collagen/hydroxyapatite composite materials. Chem Eng J. 2010;160:794–800. | ||
Albu MG, Trandafir V, Suflet DM, Chitanu GC, Budrugeac P, Titorencu I. Biocomposites based on collagen and phosphorylated dextran for bone regeneration. J Mater Res. 2012;27:1086–1096. | ||
Marques C, Ferreira JMF, Andronescu E, Ficai D, Sonmez M, Ficai A. Multifunctional materials for bone cancer treatment. Int J Nanomedicine. 2014;9:2713–2725. | ||
Ficai A, Andronescu E, Voicu G, Manzu D, Ficai M. Layer by layer deposition of hydroxyapatite onto the collagen matrix. Mater Sci Eng C Mater Biol Appl. 2009;29:2217–2220. | ||
Ilie A, Andronescu E, Ficai D, et al. New approaches in layer by layer synthesis of collagen/hydroxyapatite composite materials. Cent Eur J Chem. 2011;9:283–289. | ||
Andronescu E, Ficai A, Georgiana M, et al. Collagen-hydroxyapatite/cisplatin drug delivery systems for locoregional treatment of bone cancer. Technol Cancer Res Treat. 2013;12:275–284. | ||
Wu W, Yan C, Gan T, et al. Nuclear proteome analysis of cisplatin-treated HeLa cells. Mutat Res. 2010;691:1–8. | ||
Wagner JM, Karnitz LM. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol Pharmacol. 2009;76:208–214. | ||
Liu Y, Xing H, Han X, et al. Apoptosis of HeLa cells induced by cisplatin and its mechanism. J Huazhong Univ Sci Technolog Med Sci. 2008;28:197–199. | ||
Ficai M, Andronescu E, Ficai D, Voicu G, Ficai A. Synthesis and characterization of COLL-PVA/HA hybrid materials with stratified morphology. Colloids Surf B Biointerfaces. 2010;81:614–619. | ||
Trzaska S. Cisplatin. Chem Eng News. 2005;73(25). |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.