Back to Journals » Patient Preference and Adherence » Volume 8
An observational, retrospective, UK and Ireland audit of patient adherence to subcutaneous interferon beta-1a injections using the RebiSmart® injection device
Authors Willis H, Webster J, Larkin AM, Parkes L
Received 26 September 2013
Accepted for publication 17 December 2013
Published 12 June 2014 Volume 2014:8 Pages 843—851
DOI https://doi.org/10.2147/PPA.S54986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Helen Willis,1 Julie Webster,1 Anne Marie Larkin,2 Laura Parkes,3
1Broomfield Hospital, Chelmsford, Essex, United Kingdom; 2MySupport Nurse, Quintiles Ireland Ltd, Dublin, Ireland; 3Medical Affairs, Merck Serono Ltd, Feltham, United Kingdom
Background: Poor adherence to disease-modifying drugs is associated with an increased risk of relapse in patients with multiple sclerosis. However, adherence is difficult to assess objectively. RebiSmart® (Merck Serono SA, Geneva, Switzerland), a device for subcutaneous (sc) injection of interferon (IFN) β-1a, features an electronic injection log that can assist in objective monitoring of adherence.
Objective: To assess adherence to sc IFN β-1a injections using data from RebiSmart®.
Methods: This was a single-group, observational, retrospective audit. Adherence data were collected from patients with relapsing multiple sclerosis in the United Kingdom and Ireland who had been prescribed sc IFN β-1a and had been using RebiSmart® for a minimum of 24 months.
Results: In total, 225 patients were included in the full analysis set; 72% were in the United Kingdom, and 28% were in Ireland. Overall, the mean age was 44.1 years, and 73% were women. Patients received sc IFN β-1a 44 µg (68%) or 22 µg (32%) three times per week. Mean adherence over the course of 24 months was 95.0% (median, 99.4%), and similar values were observed across all periods. The proportion of patients with 80% or higher adherence was 92.0% at 12 months and 91.1% at 24 months.
Conclusion: High adherence to sc IFN β-1a was observed across all patient groups using RebiSmart®, according to 2-year treatment adherence data. This may be partly attributed to the expert support patients received, supplemented by routine and regular contact from the MySupport patient-support program, as well as the self-motivation of patients who persisted with treatment for 2 or more years.
Keywords: multiple sclerosis, support program, persistence, objective
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, degenerative disease of the central nervous system that can cause a wide range of debilitating symptoms affecting the physical and/or mental function of the patient.1 Although MS remains incurable, treatment with disease-modifying drugs (DMDs) can reduce the frequency of disease exacerbations and may delay disability progression.2–5 Subcutaneous (sc) interferon (IFN) β-1a (Rebif®; Merck Serono SA, Geneva, Switzerland) has been shown to be effective in improving all three key measures of efficacy (relapse rate, disability progression, and magnetic resonance imaging measures of disease) in relapsing forms of MS when administered at dosages of 44 or 22 μg three times weekly.5–7
Evidence suggests that the greatest benefit of DMD therapy is achieved when patients are treated early in the clinical course of MS;8,9 however, patients must be persistent with (continue with treatment for the prescribed duration) and adherent to (take medication in accordance with the prescribed interval and dose) their treatment regimen to achieve these benefits. Patients with MS who have poor general adherence or long gaps between treatments have been shown to be at greater risk of relapse compared with more-adherent patients.10,11 Investigators in the Global Adherence Project assessing adherence to DMDs found that compared with nonadherent patients, adherent patients had fewer problems associated with injection-site reactions, better quality of life, and fewer neuropsychological problems.12 However, rates of MS treatment discontinuation tend to be higher in clinical practice than in clinical trials. Patients in clinical trials have controlled, scheduled, regular follow-up visits and are more highly motivated to adhere to therapy.13 In a real-life setting, follow-up can be variable, and patients are exposed to many factors that can impair adherence (such as depression or denial about their disease). Notably, adherence is difficult to assess, and methods of measuring adherence can be unreliable and highly subjective.14
Injection devices are becoming commonplace for administering injectable DMDs in MS, and their use has been shown to improve patient adherence.8,15,16 RebiSmart® (Ares Trading SA, Coinsins, Switzerland) is an electronic injection device for administering sc IFN β-1a.17 This device simplifies the injection process and may help patients overcome injection-related issues. These include improving treatment satisfaction, ensuring correct injection technique, aiding patients with limited dexterity, and helping alleviate needle phobia, thereby potentially increasing adherence to treatment.15,18,19 The features of RebiSmart® include a multidose cartridge that holds the drug quantity required for 1 week; simple, step-by-step instructions; and adjustable comfort settings that allow patients to tailor injections by changing needle speed, injection speed, and time and depth of injection. RebiSmart® is also the first autoinjector for use with any MS drug that features an electronic injection log, which is able to monitor adherence objectively and may facilitate useful discussions about adherence between patients and physicians.
In the United Kingdom, patients receiving sc IFN β-1a have the drug and device supplied by a home care provider. Patients may also register with the MySupport program, which provides telephone support, text messaging, and Web site access, and can include support at home from a Merck Serono MySupport Nurse. The role of the MySupport team is to provide a postprescription support service and to collaborate with prescribing physicians and their teams, providing education, training, and expertise. The service is optional and is provided at no cost to the health service. In Ireland, patients collect their sc IFN β-1a medication from a local pharmacy and are all supported by a Merck Serono home care team, including MySupport Nurses. Patients in Ireland are provided with the Support Line number and are offered the option to register when they first access the service.
The aim of the observational RebiSmart® REtrospective ADherencE Review (READER) audit was to assess adherence to sc IFN β-1a among patients with MS (by means of a range of support services) in the United Kingdom or Ireland, using 24-month data from the RebiSmart® injection device. This study assesses the extent to which patients who persist on therapy for the 24-month period take their medication in accordance with prescribing instructions.
Methods
Audit design and patients
READER (NCT01601080)20 was a single-arm, observational, retrospective audit. RebiSmart® devices were collected at the time of replacement from patients with MS who had been prescribed sc IFN β-1a, and data on adherence during the previous 24 months and preferred comfort at final injection were made anonymous and extracted. The 24-month period was chosen because that is when the devices were routinely scheduled for replacement, thus presenting the opportunity for the evaluation of data. Patients were required to have 24 months of data to be eligible for inclusion in the study; patients with less than 24 months of data were not eligible for any of the analyses. Patients consented retrospectively, as they did not know at the start of treatment that their data might be included in this audit. Patients who were due to have their device replaced as part of standard care were asked whether they gave their consent for their adherence data to be used in the audit; they were not required to make any visits to provide data, only to provide their previous RebiSmart® device at the time of its replacement. The recruitment window for collection of returned devices was 6 months, and the recruitment target was 250 to 300 patients. The sample size was estimated from the number of devices requiring replacement. Using the assumption that the standard deviation (SD) of percentage adherence using RebiSmart® would vary from 15% to 20%, the 95% confidence interval (CI) for 250 patients would then range from 3.7% to 5.0%.
The “all patients” set (APS) included those who had administered at least a single injection of sc IFN β-1a (44 or 22 μg) using RebiSmart® during the 24-month period. The full analysis set (FAS) was defined as those patients with at least 24 months of adherence data (including any clinician-advised treatment breaks) from RebiSmart®. Patients who did not fulfill the criteria for the FAS were not included in the analyses.
Inclusion and exclusion criteria
Eligible patients were required to have relapsing MS (relapsing–remitting MS or secondary progressive MS with relapses), according to the Association of British Neurologists criteria (United Kingdom)8 and the revised 2005 McDonald criteria (Ireland),21 and to be administering sc IFN β-1a (44 or 22 μg three times weekly according to their normal dosing schedule) via the RebiSmart® injection device. Patients must have been using RebiSmart® for a minimum of 24 months before audit start and to be scheduled for a device replacement as part of their standard routine of care.
Patients were excluded if they had discontinued sc IFN β-1a before 24 months of treatment or were unwilling to give their consent. Continuation of treatment was defined by the requirement for device replacement.
Endpoints
The primary endpoint was percentage adherence measured by RebiSmart® to sc IFN β-1a injections over the course of 24 months. Percentage adherence was calculated as follows:
The number of injections received in a time period was measured as injections received between day 1 (date of first injection) and the last day of the period measured by RebiSmart®.
The secondary endpoint was percentage adherence measured by RebiSmart®, collected during months 1, 3, 6, and 12; if the patient did not have data available for the full duration of a particular period (eg, if the patient started treatment in the middle of a week), then all data to the nearest full week were used. The difference in percentage adherence between months 1–12 (12-month data) and months 12–24 (24-month data) was also assessed. Tertiary endpoints were repeated for 12-month and 24-month data and were measured as follows: the main tertiary endpoint was the difference between Medication Possession Ratio (MPR; assessed for UK-based patients only because of the method of medication distribution), calculated as
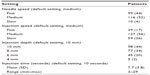
and percentage adherence at 12 and 24 months. Additional tertiary endpoints included evaluation of the effect of different variables on adherence (including age, sex, prior use of a DMD, and registration with MySupport); comparison of adherence in the United Kingdom and Ireland; and preferred comfort settings, which were obtained from the settings of the RebiSmart® device at the time of the patients’ last injection. The default comfort settings set on the device when the patient receives it include needle speed (medium), injection speed (medium), injection depth (10 mm), and injection time (10 seconds) (Table 1).
Statistical analyses
Unless otherwise stated, descriptive statistics were used throughout. Whenever specified, 95% CIs are presented for means. The software package used was SAS Version 9.1. (SAS Institute Inc., Cary, NC, USA). The primary endpoint was summarized using descriptive statistics and 95% CIs. Within-patient differences in percentage adherence between the first and second 12-month periods and within-patient difference in MPR and adherence for UK patients were normally distributed and assessed using a paired t test. Differences in percentage adherence between different patient subgroups were not normally distributed; therefore, nonparametric tests were used to test the differences between sc IFN β-1a doses (Mann–Whitney U-test) and age subgroups (Kruskal–Wallis test). The secondary and tertiary endpoints were assessed against a two-sided significance level of 5%.
Results
Baseline characteristics
In total, 230 patients were included in the APS and 225 (98%) were included in the FAS. Five patients were excluded from the APS for having less than the required 24-month adherence data (although these patients had been issued with the device for 24 months, they had not started to use it in time to provide 104 weeks of adherence data). Of the 225 patients, 161 (72%) were in the United Kingdom and 64 (28%) were in Ireland. The mean age and sex composition were as expected for this population of patients (Table 2). Sixty-six patients (29%) had previously received a DMD for MS (sc IFN β-1a without the RebiSmart® injection device or another DMD).
The majority of the patients (154 [68%]) were prescribed sc IFN β-1a 44 μg, and the remainder received 22 μg. No patients permanently switched from 44 to 22 μg during the audit; however, three patients reduced their dose to 22 μg and then titrated back up to 44 μg. All patients located in the United Kingdom, who were registered with a field nurse, were also registered with MySupport. Some patients (n=54 at 12 months; n=62 at 24 months) were registered with the MySupport Line but not with a nurse.
Endpoints
Primary endpoint (adherence at 24 months) and 12-month adherence data
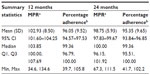
The mean percentage adherence, measured by RebiSmart®, to sc IFN β-1a injections (as defined earlier) over the course of 24 months was 95.0% (95% CI, 93.6%–96.4%) for the FAS, and the median percentage adherence was 99.4% (range, 34.9%–105.1%). The number of patients with 80% or higher adherence was 205 (91.1%; 95% CI, 86.6%–94.5%). There were 18 (8.0%) patients in the FAS with less than 80% adherence, as measured by RebiSmart®, during the first 12-month period (11/18 were women); this number increased to 20 (8.9%) during the 24-month period (14/20 were women). Within this lower than 80% adherence group, the number of patients who had previously received another DMD was nine (50.0%) at 12 months and eight (40.0%) at 24 months.
Secondary endpoints
Figure 1 shows the mean and median percentage adherence from baseline to months 1, 3, 6, and 12. Mean percentage adherence was greatest at 1 month (96.9%), but there was no notable drop in adherence by 12 months (95.4%). The number of patients with adherence of 80% or higher was also similar at the different time points observed. Mean adherence of patients with lower than 80% adherence was 63.0% (n=18; range, 30.8%–77.6%) at 12 months and 65.6% (n=20; range, 34.9%–79.2%) at 24 months. Adherence in the lower than 80% subgroup was an ad hoc analysis and included only a small number of patients. No significant difference in percentage adherence was found between the first and second 12-month periods (P=0.1906).
  | Figure 1 Box and whisker plot of percentage adherence: full analysis set over 1, 3, 6, 12, and 24 months (n=225 at all time points). |
Tertiary endpoints
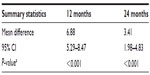
Tables 3 and 4 show MPR and percentage adherence at 12 and 24 months. At 12 months, MPR was significantly higher than adherence in patients in the United Kingdom (mean difference, 6.9%; P<0.001). By 24 months, the mean difference between MPR and adherence had reduced to 3.4% (P<0.001). Mean MPRs for patients with lower than 80% adherence at 12 months (n=10) and 24 months (n=12) were 93.5% and 92.3%, respectively. As with adherence, this was an ad hoc analysis and included only a small number of patients.
Subgroup analyses
At 12 months, mean (SD) percentage adherence was significantly higher for patients receiving sc IFN β-1a 44 μg (96.5% [9.9%]) compared with those receiving 22 μg (93.0% [13.1%]; P<0.001) (Table 5). The difference was also significant at 24 months (P=0.002). Mean adherence of patients in the United Kingdom at 12 and 24 months was 96.1% and 95.4%, respectively; for patients in Ireland, it was 93.6% and 94.2%, respectively (no significant difference between adherence in the two countries) (Table 5). No significant differences in percentage adherence between age categories were seen at 12 months (P=0.099) or 24 months (P=0.126) (Table 5). Furthermore, there were no significant differences in mean adherence at 12 or 24 months for patients registered with any MySupport services (96.2% and 95.6%) compared with those not registered with MySupport (95.6% and 94.0%) (Table 5).
Comfort settings
Final preferred comfort settings, as indicated by the settings of RebiSmart® at the time of the last injection, are shown in Table 1. Approximately half of all patients were using a setting different from the default comfort settings in place when the device was supplied.
Discussion
In general, high adherence to therapy (around 95%) was observed across patients with MS in this audit who were persistent with their treatment using RebiSmart® over a minimum of 2 years. This is considerably higher than rates reported in the literature; the Global Adherence Project12 study found that adherence over a 4-week period was 75%. In a multicenter, observational, three-wave US study of 798 patients by Treadaway et al,22 36% to 39% of patients had missed at least a single injection over the course of 4 weeks. More recently, Wicks et al23 found that up to 51% of 431 patients with relapsing–remitting MS had missed at least a single dose of their DMD during the previous month. However, direct comparisons cannot be made between the current audit and these earlier analyses22,23 as our audit only included patients who were persistent for 2 years, whereas these earlier studies monitored adherence to treatment much closer to the commencement of therapy. The results from this audit must therefore be interpreted with caution.
There was no significant difference in adherence at 24 months between patients registered with MySupport and those who were not. It should be noted that patients in the current audit were those who had persisted with treatment at 24 months. Discontinuation of treatment with IFN β, if it is to occur, is most likely in the first 6 months of therapy.24 It is probable that patients who are more likely to persist with treatment are also more likely to adhere to treatment, which may have reduced the ability of our audit to detect differences in adherence between patients who were receiving enhanced support compared with those who were not.
Patients with less than 80% adherence generally show similar characteristics to those with 80% or higher adherence; therefore, monitoring is needed, as it is difficult to identify potentially nonadherent patients. There are various reasons why patients struggle to adhere, including depression, fear of the unknown/future, adverse events, effect on quality of life, or the fact that the injections remind patients of their disease.25 The electronic features of the RebiSmart® device cannot aid adherence in these cases; however, the data from the log may help facilitate discussion between patients and their healthcare professionals. Patients with reduced adherence may need additional support to understand their personal hurdles, and monitoring using the RebiSmart® device can help identify these individuals. It has been recognized that no single intervention can improve adherence in all patients;26–28 success requires tailoring interventions to individuals, their disease, and their treatment regimen.29 Martin et al30 recognized that the healthcare provider–patient partnership remains at the core of improving adherence behaviors and suggested that participation, engagement, collaboration, negotiation, and compromise are key factors in encouraging patients to take responsibility for their treatment adherence. These partnerships foster greater patient satisfaction, improved patient adherence, and ultimately, optimal healthcare outcomes.31
Although significant differences were observed between MPR and adherence in patients in the United Kingdom at 12 and 24 months, the difference in percentage points was relatively small and is not considered to be clinically significant. MPR is known to overestimate adherence and so is not considered to be an accurate measure31 as patients need to keep additional backup stock of their medication (usually 1 month’s supply). Adherence and MPR in the less than 80% adherence group were ad hoc analyses and included only a small number of patients. The range of the percentage adherence values in this group was very wide (34.9%–79.2% at 24 months), suggesting that a group including all patients with less than 80% adherence may have been too broad; additional breakdown of these data may have proven useful. It should be noted that the range of MPR at 24 months in this less than 80% adherence group was far higher (67.3%–103.8%), supporting the theory that MPR significantly overestimates adherence. RebiSmart® is currently the only device with the capability to measure objectively treatment adherence in patients with MS. The RebiSmart® record of adherence allows the neurologist and clinical nurse specialist to make an informed decision regarding treatment; for instance, a consultant mistakenly believing that sc IFN β-1a is not working for a patient may find that the patient’s adherence is far lower than expected.32 Monitoring adherence also allows medication to be managed and waste to be minimized. Research has shown that services providing targeted support for patients starting new therapies or costly and/or difficult-to-take treatments can reduce waste, among other benefits.33
Interestingly, no effect of age on adherence levels was noted. It could be perceived that more sophisticated electronic injection devices appeal more to younger, more technology-oriented patients; however, this does not appear to be the case in this audit, and the benefits provided by the RebiSmart® device appear to be equally applicable across all of the age groups (the mean age of the audit population was 44.1 years; range, 23–67 years).
Following feedback from the MySupport Nurses who have extensive experience using the device with patients, MySupport recommends changing the needle speed to fast and the injection speed to slow for most patients. Correspondingly, around 50% of the patients changed the device settings, potentially suggesting that patients feel able and comfortable in adjusting the device to their own requirements. This is a slightly lower proportion than observed in a previous multicenter study (the RebiSmart® User Trial) of 102 patients, in which 61% changed their baseline comfort settings by 12 weeks.34
Limitations
The audit only included patients who were classified as persistent for 2 years. This is likely to represent a more motivated subgroup of patients compared with those who discontinued with treatment before this point. This may have reduced the ability of the audit to detect differences in the adherence of patients receiving support compared with those who were not. In addition, persistence was defined only in that patients were “continuing” treatment by taking delivery of a new device (if neither they themselves nor the hospital had indicated they were off treatment), rather than being defined by a specific treatment gap. There may also have been an issue of subject-selection bias, as patients who did not give consent for their data to be used may have been those with lower adherence.
No clinical data were extracted on these patients. The aim of the study was to gather adherence and patient demographic data on a wide cross-section of patients using the RebiSmart® device in the United Kingdom. It was felt that attempting to add clinical observations from patients across a large geographic area would reduce the number of patients whose devices could be audited in a reasonable time. Data on patterns of usage were not analyzed, so it is not known whether patients were continually nonadherent or whether they had major drug holidays. Although there are warnings and the ability to set reminders on the RebiSmart® device to encourage appropriate usage, patients can override these features, and therefore it is possible that patients may take doses in a nonscheduled manner (thus masking suboptimal patterns of adherence), although this is deemed unlikely.
Aggregated data were used to compare within-patient variations in adherence; however, the use of multilevel analyses would potentially have provided greater insights.
Conclusion
In general, high adherence to therapy (95%) was observed in patients with MS who were persistent with treatment for 2 years. This may be partly attributed to the support patients received from their MS nurses on an ongoing basis, supplemented by routine and regular contact from the MySupport program to maintain patient persistence to therapy. Further studies are needed to elucidate the contribution of such support services to treatment persistence. The levels of adherence observed in this audit suggest that improvements in adherence may be achieved through patient use of a variety of support services and injection devices intended to make treatment administration simpler and more comfortable.
Acknowledgments
This audit was supported by Merck Serono Ltd, UK, an affiliate of Merck KGaA, Darmstadt, Germany, and Bupa Home Healthcare. The authors thank Quanticate Ltd for data management and analysis and Lisa Tatler and Dominic Jack of Caudex Medical, Oxford, United Kingdom (supported by Merck Serono SA, a subsidiary of Merck KGaA, Darmstadt, Germany), for assistance in the preparation of this manuscript.
Disclosure
Anne Marie Larkin was an employee of Quintiles Ireland Ltd, providing MySupport Nurse support on behalf of Merck Serono in Ireland, at the time of the audit and preparation of the manuscript. Laura Parkes was an employee of Merck Serono Ltd, United Kingdom, at the time of the audit and preparation of the manuscript. The authors report no other conflicts of interest in this work.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. | |
IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):655–661. | |
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–294. | |
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–1276. | |
PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. | |
Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67(6):944–953. | |
PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001;56(12):1628–1636. | |
Association of British Neurologists. Revised (2009) Association of British Neurologists’ guidelines for prescribing in multiple sclerosis. Available from: http://www.abn.org.uk/abn/userfiles/file/ABN_MS_Guidelines_2009_Final(1).pdf. Accessed March 11, 2013. | |
Trojano M, Pellegrini F, Paolicelli D, et al; Italian Multiple Sclerosis Database Network (MSDN) Group. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol. 2009;66(4):513–520. | |
Al-Sabbagh A, Bennet R, Kozma C, Dickson M, Meletiche D. Medication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse: findings from a national managed care database. J Neurol. 2008;255(Suppl 2):S79. | |
Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2013;(2):89–100. | |
Devonshire V, Lapierre Y, Macdonell R, et al; GAP Study Group. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77. | |
Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP. Long-term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. Eur Neurol. 2008;59(3–4):131–135. | |
Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Expert Opin Drug Deliv. 2009;6(9):995–1002. | |
Devonshire VA, Verdun di Cantogno E. Review of subcutaneous interferon β-1a, delivered via the electronic self-injection device RebiSmart™, for the treatment of multiple sclerosis. Ther Deliv. 2011;2(11):1455–1465. | |
Exell S, Verdun E, Driebergen R. A new electronic device for subcutaneous injection of IFN-β-1a. Expert Rev Med Devices. 2011;8(5):543–553. | |
Lugaresi A. RebiSmart™ (version 1.5) device for multiple sclerosis treatment delivery and adherence. Expert Opin Drug Deliv. 2013;10(2):273–283. | |
Devonshire V, Feinstein A, Fortin K. Anxiety and adherence in patients with relapsing multiple sclerosis using RebiSmart for self-injection: preliminary results from the MEASURE study. J Neurol. 2012; 259(Suppl 1):S111. | |
Lugaresi A, Bellantonio B, Brescia-Morra V, et al. BRIDGE: a 12-week, multicentre, open-label, single-arm, Phase IV study of the effect of a new electronic autoinjection device on adherence to subcutaneous interferon beta-1a treatment for relapsing-remitting multiple sclerosis. Poster presented at: XLI Congress of the Italian Society of Neurology; October 23–27, 2010; Catania, Italy. | |
Merck KGaA. An Observational, Retrospective, UK & Ireland Audit of Patient Adherence to Rebif® Injections Using the RebiSmart™ Injection Device (READER). Available from: http://clinicaltrials.gov/ct2/show/NCT01601080. NLM identifier: NCT01601080. Accessed March 7, 2014. | |
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. | |
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256(4):568–576. | |
Wicks P, Massagli M, Kulkarni A, Dastani H. Use of an online community to develop patient-reported outcome instruments: the Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). J Med Internet Res. 2011;13(1):e12. | |
Tremlett HL, Oger J. Interrupted therapy: stopping and switching of the beta-interferons prescribed for MS. Neurology. 2003;61(4):551–554. | |
Lugaresi A, Florio C, Brescia-Morra V, et al; BRIDGE study group. Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurol. 2012;12:7. | |
Cheng TL, Ottolini MC, Baumhaft K, Brasseux C, Wolf MD, Scheidt PC. Strategies to increase adherence with tuberculosis test reading in a high-risk population. Pediatrics. 1997;100(2 Pt 1):210–213. | |
Hamilton GA, Toberts SJ, Johnson JM, et al. Increasing adherence in patients with primary hypertension: an intervention. Health Values. 1993;17:3–11. | |
Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36(8):1138–1161. | |
McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. | |
Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199. | |
Nau DP. Proportion of Days Covered (PDC) as a preferred method of measuring medication adherence. Available from: http://www.pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf. Accessed December 10, 2013. | |
Freedman M, Forrestal F. Treatment optimization recommendations can predict relapse rates in patients with MS: analysis of the PRISMS study data. Poster presented at: American Academy of Neurology 57th Annual Meeting; April 9–16, 2005; Miami Beach, FL. | |
Trueman P, Lowson K, Blighe A, et al. Evaluation of the scale, causes and costs of waste medicines. Available from: http://eprints.pharmacy.ac.uk/2605/1/Evaluation_of_NHS_Medicines_Waste__web_publication_version.pdf. Accessed December 10, 2013. | |
Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb study. BMC Neurol. 2010;10:28. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.