Back to Journals » Clinical Interventions in Aging » Volume 9
Aging of vestibular function evaluated using correlational vestibular autorotation test
Received 14 May 2014
Accepted for publication 19 June 2014
Published 3 September 2014 Volume 2014:9 Pages 1463—1469
DOI https://doi.org/10.2147/CIA.S67720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Li-Chun Hsieh,1,2 Hung-Ching Lin,2,3 Guo-She Lee4,5
1Institute of Brain Science, School of Medicine, National Yang-Ming University, Taipei, Taiwan; 2Department of Otolaryngology, Mackay Memorial Hospital, Taipei, Taiwan; 3Department of Audiology and Speech Language Pathology, Mackay Memorial Medical College, Taipei, Taiwan; 4Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan; 5Department of Otolaryngology, Taipei City Hospital, Ren-Ai Branch, Taipei, Taiwan
Background: Imbalance from degeneration of vestibular end organs is a common problem in the elderly. However, the decline of vestibular function with aging was revealed in few vestibular function tests such as vestibular autorotation test (VAT). In the current VAT, there are drawbacks of poor test–retest reliability, slippage of the sensor at high-speed rotations, and limited data about the effect of aging. We developed a correlational-VAT (cVAT) system that included a small, light sensor (less than 20 g) with wireless data transmission technique to evaluate the aging of vestibular function.
Material and methods: We enrolled 53 healthy participants aged between 25 and 75 years and divided them into five age groups. The test conditions were vertical and horizontal head autorotations of frequencies from 0 to 3 Hz with closed eyes or open eyes. The cross-correlation coefficient (CCC) between eye velocity and head velocity was obtained for the head autorotations between 1 Hz and 3 Hz. The mean of the CCCs was used to represent the vestibular function.
Results: Age was significantly and negatively correlated with the mean CCC for all test conditions, including horizontal or vertical autorotations with open eyes or closed eyes (P<0.05). The mean CCC with open eyes declined significantly at 55–65 years old and the mean CCC with closed eyes declined significantly at 65–75 years old.
Conclusion: Vestibular function evaluated using mean CCC revealed a decline with age, and the function of visual-vestibulo-ocular reflex declined 10 years earlier than the function of vestibulo-ocular reflex.
Keywords: vestibular autorotation test, aging, correlation analysis, gyrometry, electro-oculography
Introduction
Imbalance is a common clinical problem in the elderly. Subsequently, falls and fractures may occur. The prevalence of dizziness and vertigo has been reported to be 17% in the general population, increasing up to 39% in those over 80 years of age.1 Falls are a highly morbid and costly health condition affecting older individuals,2 of which dysfunction of the vestibular system is a risk factor.3 Measurements of gait and balance with Tinnetti score and the number of falls have shown highly significant age-related changes every year.4 Vestibular function is important for navigation, and is therefore highly relevant to the everyday lives of older people.5 Evaluating with modified Romberg test, the prevalence of vestibular dysfunction showed a significant progression with age.6 Elderly people with benign paroxysmal positional vertigo have a significantly higher recurrence rate of vertigo and need to minimize the potential morbidity of their falls.7
Vestibular dysfunction can be caused by any pathology in vestibular end organs, including the three semicircular canals and otolith organs. The peripheral vestibular system in animals and humans displays a variety of age-related changes in histology.8,9 The hair cells in crista ampullaris, macula utriculi, and macula sacculi diminish significantly with age, as revealed in human cadaveric temporal bone dissections.9 In the elderly, type I hair cells of cristae are lost more rapidly than those of the maculae.10 The quantity of otoconia in utricle and saccule reduces with age, and there is a distinct change in the shape of otoconia.9 The loss of otoconia volume is greater for macula sacculi than for macula utriculi in the elderly.8
Age-related balance disorders have been investigated, however, there is still some controversy about the decline of vestibular function during aging. Mulch and Petermann demonstrated that caloric response did change in aging,11 but Mallinson and Longridge found that vestibular function evaluated with caloric testing did not decline with age.12 Besides, the stimulation thresholds of vestibular evoked myogenic potentials (VEMP) positively correlated with age, and the response rates of VEMP using different stimuli were reduced in aging.13 However, Nguyen et al found that age did not affect cervical-VEMP and ocular-VEMP latencies or asymmetry ratios.14
Vestibular autorotation test (VAT) was first proposed by O’Leary and Davis in 1988 to evaluate vestibular functions using 18-s active sinusoidal head-turns in both horizontal and vertical planes. The test frequencies are within the physiologic range of 0.5–6.0 Hz.15 The vestibular function was presented as gain and phase by measuring eye movements and head rotations. Many previous studies have suggested that the VAT may be useful in evaluating patients with vestibulopathies, such as benign paroxysmal positional vertigo, vestibular neuritis, and Menière’s disease.16–18 However, there were limited reports about the change of vestibular function with aging evaluated by VAT. Besides, VAT is still not widely used clinically because of several weakness such as uncertain sensitivity and specificity, and poor test–retest reliability.19 Other drawbacks of VAT include a slippage of the head velocity sensor at high-frequency rotations during testing, the contributions of cervico–ocular reflex to compensate eye movements, and incompatible results between different VAT systems. Only a very limited number of published studies of VAT have investigated the effect of age and the risk of falls. In this study, we developed the correlational-VAT (cVAT) system that included a small, light sensor using wireless data transmission and new analytical methodology to establish normative data of the VAT in different age groups.
Methods
Participants
Fifty-three healthy participants, aged between 25 and 75 years, were enrolled and were divided into five age groups of 10 year intervals (25–35, 35–45, 45–55, 55–65, and 65–75 years). None of the participants had a history of upper respiratory tract infection 1 week before the test, or past history of vertigo, hearing loss, head surgery, or ear surgery. The participants with visual disturbances, oculomotor diseases, cervical spine lesions, and mental diseases were also excluded. All participants read and signed an informed consent before the test. This study was approved by the Institutional Review Board of Mackay Memorial Hospital (IRB: 11MMHIS131).
Study design
A small, light sensor (5×2×1 cm in size and less than 20 g in weight) consisted of a two-axis electronic gyrometer to detect angular velocity of the head’s pitch and yaw and two channels of electro-oculography (EOG) to catch horizontal and vertical eye movements. The maximal angular velocity of electronic gyrometry was 2000°/s and the sensitivity was 0.5 mV/°/s. The sensor was fixed at the vertex of the head with a headband, the experimental scenario is shown in Figure 1. The signals of head rotations and EOG were digitally sampled at the rate of 250 Hz, and 500 Hz, respectively. The digital resolution of signals was 16 bit. Both the signals of gyrometer and EOG were wirelessly transmitted to a laptop computer through 2.4 G Hz radiofrequency with a maximal transmission of 2.5 kbit/s, and were processed digitally with a program developed in LabVIEW 2010 (National Instruments, Austin, TX, USA).
  | Figure 1 The photography of experimental scenario (A) and sensor fixed at the vertex of head with a headband (B). |
The calibrations of gyrometry in both horizontal (yaw) and vertical (pitch) dimensions were performed by placing the sensor in an apparatus rotating at the constant speeds of 1 Hz and 2 Hz in horizontal and vertical plane, respectively. The EOG calibrations were performed by instructing the participants to sit at a distance of 100 cm in front of the projection screen. The seat was adjusted so that their eyes and the center of the projected target were in the same horizontal and vertical planes. Five cycles of 15 degree horizontal saccades and 10 degree vertical saccades were used to map the physical eye movement values.
First, the participants rotated their heads sinusoidally as fast as possible at a frequency of near 3 Hz and slowed down gradually until coming to a complete stop on the instruction of the examiner. The whole duration of each VAT was about 2 minutes. There were four test conditions, including horizontal head autorotations (head shaking) with eyes open (HO), horizontal head autorotations with eyes closed (HC), vertical head autorotations (head nodding) with eyes open (VO), and vertical head autorotations with eyes closed (VC). Each test was repeated once for data averaging and a 2 minute break was allowed between tests. In the open eye conditions of HO and VO, the participants gazed at a projected bar 1 meter in front of them. When the participants closed their eyes in HC and VC, they wore an eye cover for the duration of the test. The four test conditions were performed randomly. All participants could complete the tests successfully and without sensor slippage.
Assessment
Unlike the traditional measurements of gain and phase, the cross correlation analysis between eye velocity and head velocity was used to assess vestibular function. The cross-correlation coefficient (CCC) was defined as the cross correlation function between the velocities of eye movements and head rotations. The CCC equation for rotational velocity is provided as follows:
|
where xi is the eye velocity at time i, 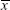 is the mean eye velocity of the analytical window, yi is the head rotational velocity at time i, and
is the mean eye velocity of the analytical window, yi is the head rotational velocity at time i, and 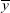 is the mean head rotational velocity of the analytical window. The CCCs were also divided into six frequency bands (0.0 to 0.5, 0.5 to 1.0, 1.0 to 1.5, 1.5 to 2.0, 2.0 to 2.5, and 2.5 to 3.0 Hz). The vestibulo-ocular reflex depends more on vestibular system in high-speed head rotations,20 therefore, we used mean CCC to evaluate the vestibular function. Mean CCC was defined as the average of the CCCs of the following four frequency bands: 1.0 to 1.5; 1.5 to 2.0; 2.0 to 2.5, and 2.5 to 3.0 Hz.
is the mean head rotational velocity of the analytical window. The CCCs were also divided into six frequency bands (0.0 to 0.5, 0.5 to 1.0, 1.0 to 1.5, 1.5 to 2.0, 2.0 to 2.5, and 2.5 to 3.0 Hz). The vestibulo-ocular reflex depends more on vestibular system in high-speed head rotations,20 therefore, we used mean CCC to evaluate the vestibular function. Mean CCC was defined as the average of the CCCs of the following four frequency bands: 1.0 to 1.5; 1.5 to 2.0; 2.0 to 2.5, and 2.5 to 3.0 Hz.
Statistical analysis
The correlation between CCC and age was analyzed using Spearman correlation analysis. One way analysis of variance (ANOVA) with post hoc least significant difference (LSD) procedure was used for multiple comparisons between different age groups. Intra-class correlation analysis was used to evaluate the test–retest reliability. The statistical analysis was carried out using SPSS 12 (SPSS Inc., Chicago, IL, USA). The results are expressed as mean ± standard error (SE) of the mean. Statistical significance was assumed if P<0.05.
Results
A typical example is showed in Figure 2. The total duration of a test was about 2 minutes. Findings of the test are displayed in Figure 2, which represented the sampling of 25 seconds in duration. Figure 2A and B show the EOG and gyrometry of VO in one 37 year-old male and one 70 year-old female, respectively. The CCCs of different frequency bands of the two participants are presented in Figure 2C and D. The CCCs of the younger participant were around 0.8 to 0.9 for the head rotations from 0.5–2.0 Hz. However, the CCCs of the aged participants were lower than the younger participants with an average of around 0.7 for the head rotations from 0.5–2.5 Hz, and the distribution of CCCs were also in a wider range.
Age was significantly and negatively correlated with mean CCC for HO (Figure 3A, ρ=−0.76, P<0.05, Spearman correlation analysis), HC (Figure 3B, ρ=−0.39, P<0.05, Spearman correlation analysis), VO (Figure 3C, ρ=−0.51, P<0.05, Spearman correlation analysis), and VC (Figure 3D, ρ=−0.28, P<0.05, Spearman correlation analysis).
The mean CCCs of HO, HC, VO, and VC in all participants are shown in Figure 4. The mean CCCs for HO, HC, VO, and VC were 0.79±0.12 (mean ± standard deviation [SD]), 0.43±0.18, 0.69±0.12, and 0.54±0.13, respectively. The variances of mean CCC for HO, HC, VO, and VC were 0.01, 0.03, 0.01, and 0.02, respectively. For HO and VO, the function of visual-vestibulo-ocular reflex (VVOR) evaluated by mean CCC decreased significantly with age, especially in the 65–75 and/or 55–65 years age groups (Figure 4A and C, P<0.05, one-way ANOVA, post hoc LSD procedure). For HC and VC, the function of vestibulo-ocular reflex (VOR) also declined significantly with age, especially in the 65–75 years age group (Figure 4B and D, P<0.05, one-way ANOVA, post hoc LSD procedure).
Intra-class correlation analysis showed that the coefficients of HO, HC, VO, and VC in the 55–65 years age group were 0.89, 0.97, 0.85, and 0.94, respectively.
Discussion
In the research, the wireless cVAT system consisting of electronic gyrometry, EOG, and correlation analysis was used to evaluate vestibular function in different age groups. The system revealed an optimal signal acquisition, minimal risk of sensor slippage at high-speed head autorotations from the advantages of wireless data transmission, lightness of the sensor, and good reliability. The function of VVOR and VOR presented with a significant decline in aging as indicated by the decrease of mean CCC. The cVAT system may be used to evaluate the changes of vestibular function following treatment and training for vestibular disorders in the future.
Dizziness is the most common complaint for patients older than 75 years, and 7% of elderly patients older than 65 years presents to primary care physicians with this symptom.21 There is higher risk of falls and fractures in the elderly. The Tinetti Performance Oriented Mobility Assessment (POMA) is widely used to determine the risk of falls in the elderly within the next year.22 The Tinetti score revealed an increase with aging.4 However, the POMA has some disadvantages, such as having a relatively complicated and time-consuming procedure and the difficulty in assessing subtle changes of training and treatment. Therefore, other measurements such as posturographic measurements of body sway23 and vestibular asymmetry by headshake test24 are used to predict the risk of falling in the elderly. But only a limited number of studies have used VAT to predict the risk of falls. However, the cVAT system in this study revealed significant decline of vestibular function in the elderly. In the future, this system may be used to evaluate the risk of falls.
Both the visual tracking system and the VOR help stabilize the visual field by making compensatory eye movements during head motion. Therefore, the CCCs with eyes open, which are synergistically operated via both the visual tracking system and VOR, showed predominance over those with eyes closed, which is when the VOR alone is operating. According to our results, the mean CCC of HO and VO declined obviously at 55–65 years old. The function of VVOR declined 10 years earlier than VOR. The VVOR evaluated by dynamic visual acuity and Gaze Stabilization test also showed a significant decline in participants older than 60.25,26 The results may imply that the vision system helps maintain balance and degenerates earlier than the vestibular system.
In our study, the vestibular function declined significantly at 65 years of age. This result is compatible with the other studies, such as the function of three semicircular canals evaluated separately with head thrust dynamic visual acuity test which declined remarkably at older than 70 years of age.25 The peak-to-peak amplitude of ocular-VEMP and cervical-VEMP also revealed a noticeable decrease in participants older than 60 years.13,14,25,27,28 In one 5 year longitudinal study including a population of participants older than 70 years, the healthy and dizzy participants both presented with a decay of vestibular function with age revealed by rotational chair tests.29
The cVAT system has some advantages, first, it uses a light, small sensor that can be easily anchored to the vertex of the head with a headband. No sensor slippage or discomfort from compression of the headband was recorded in any of the participants. Second, the EOG and head movement signals were transmitted wirelessly, thereby significantly reducing the noises from the cables. Third, in comparison with literature review,19,30,31 the variance of cVAT was smaller30 and the test–retest reliability was better by the intra-class correlations of greater than 0.85 and even as high as 0.97.19,31 Finally, existing VATs use gain and phase to represent vestibular function, where the gain is calculated as the ratio of maximal velocity of eye and head movements at that time point. In contrast, the CCC is the correlation of eye and head velocity in a time window that includes the information of both gain and phase. The signal processing and averaging of data over much more than 18 seconds may provide better accuracy than existing VATs, and the CCC may have potential in clinical applications.
There are some limitations to this cVAT system. First, artifacts could affect the acquisition of vertical EOG signals from eyelid blinking and ocular muscle activities. In our experience, using an eye cover in closed eyes testing should be able to reduce the artifacts to some extent. Second, C-spine pathology should be excluded to prevent the risk of spinal injury. Third, some participants may have difficulty in performing high-frequency autorotations greater than 2.5 Hz. According to our experiences, the complete ratio of head rotation was less than 50% at a frequency greater than 2.5 Hz, hence less information could be acquired at higher frequencies. Finally, as with traditional VAT, cVAT stimulates both ears simultaneously and the lesion side of vestibular pathologies is not easy to identify using this system.
Conclusion
In conclusion, the decline of visual-vestibular function with aging can be detected by the cVAT system based on correlational analysis of EOG and gyrometry of head rotations. The vision-assisted vestibular function seemed to degenerate 10 years earlier than the vestibular system alone. Further studies are needed to explore the applications of cVAT in the prediction of falls, measurement of visual-associated vestibular function, and vestibular disorders.
Acknowledgment
This work was supported by a grant from the National Science Council, Taiwan (NSC 102-2314-B-010-029).
Disclosure
The authors have no conflicts of interest to disclose.
References
Davis A, Moorjani P. The epidemiology of hearing and balance disorders. In: Luxon ML, Furmann IM, Martini A, Stephens D, Dunitz M, editors. Textbook of Audiological Medicine. London: Martin Dunitz; 2003:89–99. | ||
Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348(1):42–49. | ||
Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. | ||
Baloh RW, Ying SH, Jacobson K. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60(6):835–839. | ||
Allen GL, Kirasic KC, Rashotte MA, Haun DB. Aging and path integration skills: kinesthetic and vestibular contributions to way finding. Percept Psychophys. 2004;66(1):117–179. | ||
Agrawal Y, Carey JP, Della SCC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. | ||
Prokopakis E, Vlastos I, Tsagournisakis M, Christodoulou P, Kawauchi H, Velegrakis G. Canalith repositioning procedures among 965 patients with benign paroxysmal positional vertigo. Audiol Neurotol. 2013;18(2):83–88. | ||
Igarashi M, Saito R, Mizukoshi K, Alford BR. Otoconia in young and elderly persons: a temporal bone study. Acta Otolaryngol Suppl. 1993;504:26–29. | ||
Walther LE, Westhofen M. Presbyvertigo-aging of otoconia and vestibular sensory cells. J Vestib Res. 2007;17(2–3):89–92. | ||
Rauch SD, Velazquez-Villasenor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann N Y Acad Sci. 2001;942:220–227. | ||
Mulch G, Petermann W. Influence of age on results of vestibular function tests. Review of literature and presentation of caloric test results. Ann Otol Rhinol Laryngol Suppl. 1979;88(2 Pt 2 Suppl 56):1–17. | ||
Mallinson AI, Longridge NS. Caloric response does not decline with age. J Vestib Res. 2004;14:393–396. | ||
Janky KL, Shepard N. Vestibular evoked myogenic potential (VEMP) testing: normative threshold response curves and effects of age. J Am Acad Audiol. 2009;20(8):514–522. | ||
Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. | ||
O’Leary DP, Davis LL. Spectral analysis of low-frequency, active-head vestibulo-ocular reflex responses. J Vestib Res. 1998;8(4):313–324. | ||
Belafsky P, Gianoli G, Soileau J, Moore D, Davidowitz S. Vestibular autorotation testing in patients with benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2000;122(2):163–167. | ||
López Escamez JA, Molina MI, Zapata C, et al. Respuesta oculomotora al test de autorrotación cefálica vertical en pacientes con vértigo posicional paroxístico benigno del conducto posterior. [Oculomotor response to the vertical cephalic autorotatory test in patients with benign paroxistic positional vertigo of the posterior canal]. Acta Otorrinolaringol Esp. 2006;57(5):210–216. Spanish. | ||
Nachum Z, Gordon CR, Shahal B, Spitzer O, Shupak A. Active high-frequency vestibulo-ocular reflex and seasickness susceptibility. Laryngoscope. 2002;112(1):179–182. | ||
Blatt PJ, Schubert MC, Roach KE, Tusa RJ. The reliability of the Vestibular Autorotation Test (VAT) in patients with dizziness. J Neurol Phys Ther. 2008;32(2):70–79. | ||
Demer JL, Crane BT, Tian JR, Wiest G. New tests of vestibular function. Ann N Y Acad Sci. 2001;942:428–445. | ||
Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med. 2001;134(9 Pt 2):823–832. | ||
Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. | ||
Chiba H, Ebihara S, Tomita N, Sasaki H, Butler JP. Differential gait kinematics between fallers and non-fallers in community-dwelling elderly people. Geriatr Gerontol Int. 2005;5(2):127–134. | ||
Ekvall HE, Magnusson M. Vestibular asymmetry predicts falls among elderly patients with multi-sensory dizziness. BMC Geriatr. 2013;13(1):77. | ||
Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33(5):832–839. | ||
Honaker JA, Shepard NT. Age effect on the Gaze Stabilization test. J Vestib Res. 2010;20(5):357–362. | ||
Basta D, Todt I, Ernst A. Characterization of age-related changes in vestibular evoked myogenic potentials. J Vestib Res. 2007;17(2–3):93–98. | ||
Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001;112(11):1971–1979. | ||
Baloh RW, Enrietto J, Jacobson KM, Lin A. Age-related changes in vestibular function: a longitudinal study. Ann N Y Acad Sci. 2001;942:210–219. | ||
Corvera J, Corvera-Behar G, Lapilover V, Ysunza A. Evaluation of the Vestibular Autorotation Test (VAT) for Measuring Vestibular Oculomotor Reflex in Clinical Research. Arch Med Res. 2000;31(4):384–387. | ||
Guyot J, Psillas G. Test-retest reliability of vestibular autorotation testing in healthy subjects. Otolaryngol Head Neck Surg. 1997;117(6):704–707. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.