Back to Journals » Journal of Pain Research » Volume 7
A randomized clinical study of the heated lidocaine/tetracaine patch versus subacromial corticosteroid injection for the treatment of pain associated with shoulder impingement syndrome
Authors Radnovich R, Trudeau J, Gammaitoni AR
Received 26 February 2014
Accepted for publication 7 June 2014
Published 9 December 2014 Volume 2014:7 Pages 727—735
DOI https://doi.org/10.2147/JPR.S63118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Richard Radnovich,1 Jeremiah Trudeau,2 Arnold R Gammaitoni3
1Injury Care Medical Center, Boise, ID, USA; 2Analgesic Solutions, Natick, MA, USA; 3Nuvo Research Inc., West Chester, PA, USA
Background: Treatment for pain due to shoulder impingement syndrome (SIS) typically begins conservatively with nonsteroidal anti-inflammatory drugs and physical therapy and can include subacromial injection of corticosteroids, particularly in patients unresponsive to conservative measures. The heated lidocaine/tetracaine (HLT) patch has been reported to reduce SIS pain in a small case series.
Methods: This was a prospective, randomized, open-label clinical trial in which adult patients with SIS pain lasting at least 14 days, with an average intensity of ≥4 on a 0–10 scale (0= no pain, 10= worst pain) were randomized to treatment with the HLT patch or a single subacromial injection of triamcinolone acetonide (10 mg). Patients in the HLT patch group applied a single HLT patch to the shoulder for 4 hours twice daily, with a 12-hour interval between treatments during the first 14 days, and could continue to use the patch on an as-needed basis (up to twice daily) during the second 14-day period. No treatment was allowed in the final 14-day period. At baseline and at days 14, 28, and 42, patients rated their pain and pain interference with specific activities (0–10 scale).
Results: Sixty patients enrolled in the study (average age =51 years, range 18–75, n=21 female). Average pain scores declined from 6.0±1.6 at baseline to 3.5±2.4 at day 42 in the HLT patch group (n=29, P<0.001) and from 5.6±1.2 to 3.2±2.6 in the injection group (n=31, P<0.001). Similar improvements were seen in each group for worst pain; pain interference with general activity, work, or sleep; and range of motion. No significant between-group differences were seen for any pain or pain interference scores at any time point.
Conclusion: These results suggest that short-term, noninvasive treatment with the HLT patch has similar efficacy to subacromial corticosteroid injections for the treatment of pain associated with SIS.
Keywords: shoulder impingement syndrome, corticosteroids, heated lidocaine/tetracaine patch, pain
Introduction
Shoulder impingement syndrome (SIS), which is also referred to as subacromial impingement syndrome1 and rotator cuff disease,2 is a common cause of shoulder pain and dysfunction.1,3 The symptoms of SIS primarily include pain and limitation in the range of motion. These symptoms are thought to be caused by an impingement of the rotator cuff tendon under the acromion,4 although other mechanisms may contribute, eg, bursitis,5 tendinitis,6 rotator cuff tear,6 and rotator cuff tendinopathy.7 The biological basis of the pain of SIS is not well understood, but the histopathological changes in the rotator cuff of patients with SIS are similar to the changes seen in other overuse tendinopathies.8 Therefore the pathophysiological basis of pain in SIS might be similar to those pain mechanisms (ie, ion channel and glutamate signaling) reported for other conditions.
Treatment of SIS typically begins with conservative therapy, including oral nonsteroidal anti-inflammatory drugs (NSAIDs)9,10 and supervised physical therapy,11 with the goal of reducing pain and improving strength and function.12 About two-thirds of patients respond favorably to NSAIDs and physical therapy,12 and a recent meta-analysis of exercise programs concluded that this strategy reduced pain and increased function in the short-term.13 In patients with persistent symptoms, subacromial injections of lidocaine and a corticosteroid may be indicated to reduce pain and thus allow physical therapy to continue,10 although meta-analyses of the use of corticosteroids in SIS have failed to reveal clear evidence of benefit.9,14
The results of a recent pilot study15 and case reports16 suggest that the heated lidocaine/tetracaine (HLT) patch may represent an alternative conservative treatment for pain associated with SIS. The HLT patch (Synera®; Galen US Inc., Souderton, PA, USA) contains a eutectic mixture of lidocaine (70 mg) and tetracaine (70 mg) with an integrated oxygen-activated heating component.17 These two local anesthetics may exert their effect by targeting local ion and glutamate channels, thereby reducing peripheral pain signaling. The heating component, which is activated during administration by exposure of the HLT patch to air, has been shown to enhance the rate of delivery of lidocaine in a pharmacokinetic study.18 The depth and duration of dermal anesthesia reported in a controlled study of the HLT patch suggests that it may be effective in controlling pain in superficial musculoskeletal structures.19 In this report, we describe a randomized clinical study comparing the HLT patch with subacromial corticosteroid injections for treatment of SIS.
Materials and methods
Study ethics
The protocol was reviewed and approved by a local institutional review board. All study participants provided written informed consent prior to enrolling in the study and engaging in any study activities. The study was conducted in accordance with the World Medical Association, Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, and Title 21 of the Code of Federal Regulations Parts 50, 54, 56, and 312.
Patient population
Patients ≥18 years old with unilateral pain associated with SIS of at least 2 weeks’ duration were eligible to enroll in the study. Other inclusion criteria were having tenderness at the site of the rotator cuff tendons, having positive Hawkins’ and Neer’s signs,20 and reporting an average pain intensity of 4 on an 11-point numerical pain intensity scale (NPIS) (0= no pain, 10= worst pain imaginable) over the previous 24 hours. Reasons for exclusion included the use of any topically applied medication on the target treatment area within the 14 days preceding day 1 (prohibited medications included NSAIDs, menthol, methyl salicylate, local anesthetics [including Lidoderm®; Endo Pharmaceuticals Inc., Malvern, PA, USA], and steroids), use of any injected medication (eg, local anesthetics or steroids) within 60 days preceding day 1, filing of a disability claim or currently receiving disability payments for SIS, use of any Class I antiarrhythmic drugs, or participation in a clinical trial of an investigational drug within 30 days before screening.
Study design
This was a randomized, open-label study (Clinicaltrials.gov: NCT01544283). On day 1, patients were screened for eligibility. In addition to recording baseline average pain intensity over the previous 24 hours, patients rated their worst pain over the previous 24 hours using the 11-point NPIS. Baseline pain interference scores were recorded for general activity, normal work, and sleep, using an 11-point scale (0= no interference, 10= complete interference). Baseline shoulder ranges of motion (internal rotation and abduction) were measured with a goniometer.
Enrolled patients were randomized into two groups. In the HLT patch group, patients applied a single HLT patch to the affected shoulder twice daily for a 14-day period. Each patch was removed 4 hours after application, and applications were separated by about 12 hours. During the second 14-day period, patients in the HLT patch group were allowed to use the patch as needed up to twice daily as described above. This pragmatic dosing was chosen because most patients who treat acute musculoskeletal pain do so for a limited time until pain subsides and function improves. No use of the HLT patch was allowed during the final 14-day period to determine the durability of effect. In the injection group, patients received a single injection of 1 mL of a 10 mg/mL suspension of triamcinolone acetonide (Kenalog®-10; Bristol-Myers Squibb, New York, NY, USA) into the subacromial space of the affected shoulder. The injection was performed using a lateral approach with palpitation of bony landmarks guiding needle placement.
All patients were given a study diary and recorded their average pain intensity over the previous 24 hours each evening, using the 11-point NPIS. In addition, patients recorded their use of rescue medication for pain of SIS each evening. Acetaminophen supplied by the study investigators could be used as rescue medication for pain associated with SIS during the study. However, any patient who used acetaminophen for shoulder pain on two consecutive days was considered a treatment failure.
On days 14, 28, and 42, patients were asked to complete the Patient Global Assessment of Satisfaction (PGAS) and the Patient Global Impression of Change (PGIC) scales. The PGAS is a 5-point scale ranging from 0 (very dissatisfied) to 4 (very satisfied). The PGIC is a 7-point scale ranging from 1 (very much worse) to 7 (very much improved). Shoulder range of motion was measured at these study visits, and pain intensity and pain interference scores were also recorded.
Safety
Adverse events (AEs) were recorded at each study visit and during a telephone call on day 7. The skin at the HLT patch application site was assessed at study visits on days 14, 28, and 42, using a 5-point scale (0= no erythema, 1= very slight erythema, 2= well-defined erythema, 3= moderate to severe erythema, 4= severe erythema [beet redness] to slight eschar formation [injuries in depth]).
Data analysis
All patients who enrolled in the study and received a subacromial injection of corticosteroid or at least one HLT patch were included in the intent-to-treat and safety populations. In the intent-to-treat population, all post-baseline pain or pain interference scores for patients who were withdrawn from the study, violated the protocol, or were deemed treatment failures were imputed as baseline observations carried forward. The primary efficacy analysis (change in average pain score) was conducted on the intent-to-treat population. All statistical comparisons were conducted with a Student’s t-test. A Bonferroni correction was employed to adjust for multiple comparisons. P<0.05 was considered statistically significant. Based on an anticipated standard deviation for change in average pain scores of about 2.5, a total of 30 patients per group should result in an estimated 90% power to detect a difference of 2.0 in change of average pain score between groups. All data are expressed as mean ± standard deviation.
Results
A total of 60 patients enrolled in the study (Figure 1). Twenty-nine patients were randomized to the HLT patch group, and 31 patients were randomized to the injection group. The baseline characteristics of the patients are presented in Table 1. Twenty-three patients in each group completed the study without a protocol violation or use of acetaminophen for shoulder pain on two consecutive days. In the HLT patch group, two patients (7%) were deemed treatment failures due to excessive acetaminophen use, three patients (10%) were withdrawn due to protocol violation (improper patch use), and one patient (3%) withdrew due to an AE (increased shoulder pain). In the injection group, two patients (6%) were lost to follow-up, and six patients (19%) were deemed treatment failures due to use of acetaminophen for shoulder pain on two consecutive days. During days 14 to 28, the patients in the HLT patch group used an average of approximately one patch per day.
  | Figure 1 CONSORT flow diagram. |
Efficacy
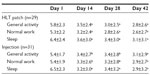
Baseline “average pain” and “worst pain” scores were similar in each group (Table 1). Both treatment groups demonstrated similar decreases in pain scores during the study (Figure 2). On day 42, 66% of patients in the HLT patch group demonstrated a clinically meaningful reduction (≥ 30% reduction in average pain score) compared with 55% of patients in the injection group (Figure 3). The number of patients who were designated as treatment failures due to two consecutive days of acetaminophen rescue use (an indirect measure of efficacy) was three times higher in the injection group than in the HLT patch group (six patients in the injection group versus two patients in the HLT patch group). Patients in both groups demonstrated similar reductions in pain interference scores during the study (Table 2).
  | Table 1 Baseline patient characteristics |
Patient assessments of treatment
PGAS
On day 42, 26 of the 29 (90%) patients in the HLT patch group reported being “satisfied” (n= 11) or “very satisfied” (n= 15) with the treatment. Three patients expressed “no preference”. In the injection group, 26 of 31 patients (84%) reported being “satisfied” (n= 5) or “very satisfied” (n= 21). Two patients expressed “no preference”, and one patient each reported being “dissatisfied” or “very dissatisfied”. One patient in the injection group did not provide a response. The distribution of responses on days 14 and 28 in both groups was similar to that observed on day 42.
PGIC
In the HLT patch group, 16 of 29 (55%) patients reported being “very much improved” (n= 5) or “much improved” (n= 11) on day 42. In the injection group, 20 of 31 patients (65%) reported being “very much improved” (n= 11) or “much improved” (n= 9). The distribution of responses for the PGAS and PGIC on days 14 and 28 in both groups were similar to those observed on day 42.
Shoulder range of motion
The effect of the treatment on shoulder range of motion is shown in Figure 4. In the HLT patch group, internal rotation increased from 36°±27° at baseline to 56°±23° after 14 days of treatment. Abduction increased from 91°±30° at baseline to 124°±23° after 14 days of treatment. These improvements were sustained through day 42 and were comparable in magnitude to those observed in the injection group. Figure 5 shows the cumulative response of shoulder range of motion from baseline to day 42. In the HLT patch group, 38% of patients achieved ≥80° internal rotation, and 14% achieved ≥170° abduction at day 42. In the injection group, the proportions of patients who achieved ≥80° internal rotation and ≥170° abduction were 23% and 23%, respectively.
  | Figure 4 Effect of treatment on shoulder range of motion. |
  | Figure 5 Cumulative shoulder range of motion at baseline and day 42. |
Safety
Twenty-seven treatment-emergent AEs were reported by 23 patients in the HLT patch group. Erythema was the most common treatment-emergent AE (n=22). All cases of erythema were considered probably or definitely related to treatment and were mild to moderate in severity. A mild bullous reaction at the application site was observed in one patient. In the injection group, ten treatment-emergent AEs were reported in five patients. All were mild or moderate in severity, and none were deemed to be related to study treatment.
Discussion
This randomized study comparing a pragmatic dosing regimen using the HLT patch with a single subacromial injection of corticosteroid for the treatment of SIS resulted in a similar reduction in pain scores (Figure 2) and pain interference scores (Table 2), and improvement in shoulder range of motion (Figure 4), in both treatment groups. Improvement in these parameters was seen as soon as 14 days after initiating treatment and persisted through day 42. The magnitude of pain relief was clearly within the range that is considered to be clinically important. At day 42, for example, 66% of the patients in the HLT patch group and 55% of the patients in the injection group experienced ≥30% reduction in average pain score (Figure 3).
Subacromial injections of corticosteroids are commonly used as an initial conservative treatment of SIS.1,9,21 Systematic reviews and meta-analyses have generally concluded that injections are effective, at least in the short term,21 although controversy regarding the quality of the evidence exists.9,14 Presumably, corticosteroid injections exert their benefit in the treatment of SIS by their anti-inflammatory properties, although the evidence of an inflammatory component to the pain of SIS is conflicting.5,22,23 Further use of injectable corticosteroids is not without risk, as previous reports of tendon rupture, subcutaneous atrophy, and articular cartilage changes have been documented.24
In this study, the HLT patch provided a noninvasive, targeted treatment that resulted in substantial reduction in pain, as well as an improvement in function, compared with baseline. Although the exact mechanism of the HLT patch for alleviating pain in SIS patients remains to be determined, there are biochemical phenomena described in common with other tendinopathies that may offer clues into potential mechanisms. These include collagen loss and disorganization and mucoid degeneration,22,25 but the biological mechanisms that contribute to pain in these conditions remain to be fully elucidated. Elevations of prostaglandin E2 were not found in microdialysis samples taken from the patellar tendon of patients with painful jumper’s knee compared with samples from unaffected subjects, an observation that calls into question the role of inflammation as a major cause of pain.26 Other biochemical mediators, like neurotransmitter glutamate, may influence nociceptors, and high levels of glutamate have been identified by microdialysis in patellar and Achilles tendinopathies.26 In addition, increased expression of the glutamate receptor, N-methyl-D-aspartate (NMDA) receptor type 1, has been found in biopsy samples from patients with patellar tendinopathy but not from control subjects.27,28 Both lidocaine and tetracaine inhibit the NMDA receptor, with tetracaine being the most potent of the local anesthetics at inhibiting NMDA receptors;29 thus, it is possible that beneficial effects of the HLT patch in painful SIS is mediated in part by blockade of this receptor.
The HLT patch is approved in the US and Europe with an indication for providing dermal anesthesia for superficial venous access and minor dermatologic procedures.17 Wallace et al found that following a 30-minute application of the HLT patch, pain sensation in response to a needle stick was eliminated to a mean depth of 8.22 mm, with the largest anesthetic effect occurring 60 minutes after removal of the HLT patch.19 The longer application times employed in the present study might be expected to result in deeper penetration of lidocaine and tetracaine. Although no direct evidence of the transdermal transit of lidocaine and tetracaine to underlying subcutaneous structures during in vivo application of the HLT patch has been reported, Sekiya et al reported that the topical application of the ketoprofen patch resulted in rapid and sustained delivery to underlying tendon and muscle without substantial increases in plasma concentrations.30 Similar local delivery of lidocaine and tetracaine to rotator cuff tendons would in part explain the results observed in the present study.
Study limitations
Although the study randomized patients to the two different treatment groups, it remained open-label. No placebo-treated control group was included, and therefore the contribution of a placebo response cannot be quantified in either treatment group.
Conclusion
The HLT patch was utilized with a short-term pragmatic dosing schedule, which was intended to reflect real-world use of analgesics by patients with acute musculoskeletal pain (fixed dosing for 2 weeks followed by as-needed dosing for the subsequent 2 weeks), and resulted in a sustained benefit lasting at least 6 weeks. This beneficial response was achieved by a noninvasive approach and was similar to that observed in the group of patients who were treated with a single subacromial corticosteroid injection. Based on these results, the HLT patch may represent an alternative initial conservative treatment for SIS that targets different mechanisms than the commonly used agents (ie, NSAIDs and corticosteroids), and further study is warranted.
Acknowledgments
The authors would like to acknowledge Thomas Marriott, PhD, for his careful review of the data and the manuscript. This study was sponsored by Nuvo Research, Inc. Professional medical writing and editing assistance was paid for by Nuvo Research, Inc., and was provided by Edward Weselcouch, PhD, and Diana Talag, MS, ELS, of PharmaWrite (Princeton, NJ). This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines”.
Disclosure
Nuvo Research, Inc., provided support for this study. Arnold Gammaitoni was formerly an employee of Nuvo Research. Jeremiah Trudeau is a paid consultant for Nuvo Research. Richard Radnovich has been a consultant for and has received research funding from Nuvo Research. The HLT patch is marketed in the United States by Galen US Inc. as Synera®. The authors have no other conflicts of interest in this work.
References
Harrison AK, Flatow EL. Subacromial impingement syndrome. J Am Acad Orthop Surg. 2011;19(11):701–708. | |
Del Buono A, Oliva F, Longo UG, et al. Metalloproteases and rotator cuff disease. J Shoulder Elbow Surg. 2012;21(2):200–208. | |
van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54(12):959–964. | |
Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41–50. | |
Blaine TA, Kim YS, Voloshin I, et al. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Shoulder Elbow Surg. 2005;14(Suppl 1):84S–89S. | |
Meislin RJ, Sperling JW, Stitik TP. Persistent shoulder pain: epidemiology, pathophysiology, and diagnosis. Am J Orthop (Belle Mead NJ). 2005;34(Suppl 12):5–9. | |
Lewis JS. Rotator cuff tendinopathy/subacromial impingement syndrome: is it time for a new method of assessment? Br J Sports Med. 2009;43(4):259–264. | |
Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27(6):393–408. | |
van der Sande R, Rinkel WD, Gebremariam L, Hay EM, Koes BW, Huisstede BM. Subacromial impingement syndrome: effectiveness of pharmaceutical interventions–NSAIDs, corticosteroid or other injections. A systematic review. Arch Phys Med Rehabil. 2013;94(5): 961–976. | |
Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447–457. | |
Senbursa G, Baltaci G, Atay A. Comparison of conservative treatment with and without manual physical therapy for patients with shoulder impingement syndrome: a prospective, randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):915–921. | |
Morrison DS, Frogameni AD, Woodworth P. Non-operative treatment of subacromial impingement syndrome. J Bone Joint Surg Am. 1997;79(5):732–737. | |
Hanratty CE, McVeigh JG, Kerr DP, et al. The effectiveness of physiotherapy exercises in subacromial impingement syndrome: a systematic review and meta-analysis. Semin Arthritis Rheum. 2012;42(3):297–316. | |
Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain [review]. Cochrane Database Syst Rev. 2003;(1):CD004016. | |
Radnovich R, Marriott TB. Utility of the heated lidocaine/tetracaine patch in the treatment of pain associated with shoulder impingement syndrome: a pilot study. Int J Gen Med. 2013;6:641–646. | |
Radnovich R. Heated lidocaine-tetracaine patch for management of shoulder impingement syndrome. J Am Osteopath Assoc. 2013;113(1):58–64. | |
Synera® (lidocaine 70 mg and tetracaine 70 mg) topical patch [prescribing information]. Souderton, PA: Galen US Inc.; 2013. | |
Marriott TB, Charney MR, Stanworth S. Effects of application durations and heat on the pharmacokinetic properties of drug delivered by a lidocaine/tetracaine patch: a randomized, open-label, controlled study in healthy volunteers. Clin Ther. 2012;34(10):2174–2183. | |
Wallace MS, Kopecky EA, Ma T, Brophy F, Campbell JC. Evaluation of the depth and duration of anesthesia from heated lidocaine/tetracaine (Synera) patches compared with placebo patches applied to healthy adult volunteers. Reg Anesth Pain Med. 2010;35(6):507–513. | |
Wilson JJ, Best TM. Common overuse tendon problems: A review and recommendations for treatment. Am Fam Physician. 2005;72(5): 811–818. | |
Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract. 2005;55(512):224–228. | |
Khan KM, Cook JL, Maffulli N, Kannus P. Where is the pain coming from in tendinopathy? It may be biochemical, not only structural, in origin. Br J Sports Med. 2000;34(2):81–83. | |
Voloshin I, Gelinas J, Maloney MD, O’Keefe RJ, Bigliani LU, | |
Min KS, St Pierre P, Ryan PM, Marchant BG, Wilson CJ, Arrington ED. A double-blind randomized controlled trial comparing the effects of subacromial injection with corticosteroid versus NSAID in patients with shoulder impingement syndrome. J Shoulder Elbow Surg. 2013;22(5):595–601. | |
Lewis JS. Rotator cuff tendinopathy. Br J Sports Med. 2009;43(4): 236–241. | |
Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19(5):881–886. | |
Schizas N, Lian Ø , Frihagen F, Engebretsen L, Bahr R, Ackermann PW. | |
Schizas N, Weiss R, Lian O, Frihagen F, Bahr R, Ackermann PW. Glutamate receptors in tendinopathic patients. J Orthop Res. 2012; | |
Sugimoto M, Uchida I, Mashimo T. Local anaesthetics have different mechanisms and sites of action at the recombinant N-methyl-D-aspartate (NMDA) receptors. Br J Pharmacol. 2003;138(5):876–882. | |
Sekiya I, Morito T, Hara K, et al. Ketoprofen absorption by muscle and tendon after topical or oral administration in patients undergoing anterior cruciate ligament reconstruction. AAPS PharmSciTech. 2010;11(1):154–158. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.