Back to Journals » International Journal of Nanomedicine » Volume 9 » Supplement 2
A 90-day study of subchronic oral toxicity of 20 nm, negatively charged zinc oxide nanoparticles in Sprague Dawley rats
Authors Park H, Shin S, Meang E, Hong J, Park J, Kim S, Koh S, Lee S, Jang D, Lee JY, Sun Y, Kang JS, Kim Y, Kim M, Jeong J, Lee JK, Son W, Park J, An SSA
Received 20 November 2013
Accepted for publication 2 June 2014
Published 15 December 2014 Volume 2014:9(Supplement 2) Pages 79—92
DOI https://doi.org/10.2147/IJN.S57926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Hark-Soo Park,1 Sung-Sup Shin,1 Eun Ho Meang,1 Jeong-sup Hong,1 Jong-Il Park,1 Su-Hyon Kim,1 Sang-Bum Koh,1 Seung-Young Lee,1 Dong-Hyouk Jang,1 Jong-Yun Lee,1 Yle-Shik Sun,1 Jin Seok Kang,2 Yu-Ri Kim,3 Meyoung-Kon Kim,3 Jayoung Jeong,4 Jong-Kwon Lee,4 Woo-Chan Son,5 Jae-Hak Park6
1General Toxicology Team, Korea Testing and Research Institute, Seoul, Korea; 2Department of Biomedical Laboratory Science, Namseoul University, Cheonan, Korea; 3Department of Biochemistry and Molecular Biology, Korea University Medical School and College, Seoul, Korea; 4National Institute of Food and Drug Safety Evaluation, Seoul, Korea; 5Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea; 6Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea
Purpose: The widespread use of nanoparticles (NPs) in industrial and biomedical applications has prompted growing concern regarding their potential toxicity and impact on human health. This study therefore investigated the subchronic, systemic oral toxicity and no-observed-adverse-effect level (NOAEL) of 20 nm, negatively charged zinc oxide (ZnOSM20(-)) NPs in Sprague Dawley rats for 90 days.
Methods: The high-dose NP level was set at 500 mg/kg of bodyweight, and the mid- and low-dose levels were set at 250 and 125 mg/kg, respectively. The rats were observed during a 14-day recovery period after the last NP administration for the persistence or reduction of any adverse effects. Toxicokinetic and distribution studies were also conducted to determine the systemic distribution of the NPs.
Results: No rats died during the test period. However, ZnOSM20(-) NPs (500 mg/kg) induced changes in the levels of anemia-related factors, prompted acinar cell apoptosis and ductular hyperplasia, stimulated periductular lymphoid cell infiltration and excessive salivation, and increased the numbers of regenerative acinar cells in the pancreas. In addition, stomach lesions were seen at 125, 250, and 500 mg/kg, and retinal atrophy was observed at 250 and 500 mg/kg. The Zn concentration was dose-dependently increased in the liver, kidney, intestines, and plasma, but not in other organs investigated.
Conclusion: A ZnOSM20(-) NP NOAEL could not be established from the current results, but the lowest-observed-adverse-effect level was 125 mg/kg. Furthermore, the NPs were associated with a number of undesirable systemic actions. Thus, their use in humans must be approached with caution.
Keywords: negative charge, oral toxicity study, rat, ZnO
Introduction
Nanotechnology has developed at an extremely rapid pace over the past several years in numerous fields of research (eg, regenerative medicine, cell biology, pharmaceuticals, and cosmeceuticals). However, the uptake of nanomaterials into the body through various routes of administration (ie, dermal, inhalation, injection, and oral) may pose risks to human health. Careful studies addressing the concentrations, dosages, and reactivity of these materials are therefore required. In this regard, concern about the health and safety aspects of nanoparticles (NPs) and other nanomaterials is increasing in industrial as well as biomedical applications.1,2 Zinc oxide (ZnO) NPs have been synthesized recently by gas and liquid phase synthesis and sol-gel processing for varistor devices.3 Also, the precursor of benzylamine and Zn acetylacetonate have been used synthesizing ZnO NPs based on non-aqueous sol-gel method.4 NPs have the unique characteristic of being miscible with water. They also have a large surface area, as well as excellent reactivity with liquids, gasses, and similarly sized biological molecules.5,6 NPs are atomic or molecular aggregates with at least one dimension between 1 and 100 nm, which modifies their physicochemical properties compared with the bulk materials.7–9 It is worth noting, however, that NPs can be made from a variety of bulk materials, and that their actions depend on both their chemical composition and the size and/or shape of the individual particles.10
Nanomaterials, including those composed of titanium dioxide (TiO2) and ZnO, have been used in cosmetics and pharmaceuticals for many years.11 ZnO NPs are capable of absorbing ultraviolet rays and transmitting visible light, and thus their use is exploited in numerous personal care products, including sunscreens, foundations and other topical lotions, reflective/glare-proof coatings, and paint. Nevertheless, apprehension about the possible danger of NPs in the body continues.12 An oral exposure can be an occupational and environmental route, resulting from ingestion of food and water, and swallowing of inhaled particles. Studies of iridium and fullerene particles in rats have shown minimal systemic absorption.13,14 For example, negatively charged latex NPs can diffuse across the mucus layer, interact with epithelial cells, and be transported into other organs in rats.15 In addition, a nanoparticulate extract derived from Brassica rapa (turnip) was recently shown to exert anti-hepatofibrogenic effects in the liver.16 These observations indicate that the systemic actions of NPs are not predictable and, indeed, may be harmful. Nanomaterials and NPs have toxicity in the respiratory, nervous, digestive, immune, and circulatory systems. The toxicity of NPs is related to the dose, size, surface area, particle chemistry, crystalline structure, and surface coating.17 The toxicity of ZnO NPs in a recent 14-day in vivo study demonstrated histopathologic lesions in liver, kidney, lung, spleen, and pancreas and elevation of liver dysfunction factor.18
The current study explored the latent toxicity of negatively charged ZnO NPs with a diameter of 20 nm (ZnOSM20(−) NPs). ZnO NPs were chosen because of their widespread use in diverse applications, as noted above. Furthermore, size and surface-coating modifications can influence the toxicity of ZnO NPs in vitro. For these reasons, we conducted a novel 90-day repeated dose, subchronic oral toxicity investigation of ZnOSM20(−) NPs in Sprague Dawley (SD) rats to ascertain their systemic toxicity and the no-observed-adverse-effect level (NOAEL) or lowest-observed-adverse-effect level (LOAEL) in vivo.
Materials and methods
Test and control materials
ZnO NPs (Product name: Ultra fine Zinc Oxide ZnO-310) were purchased from Sumitomo Osaka Cement Co., Ltd (Tokyo, Japan). The crystalline structure and the size of ZnO NPs were analyzed in this study by X-ray diffraction and Fourier transform spectroscopy. The average diameter was 29±3 nm in deionized water.19 The vehicle control corresponded to 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES)-citrate buffer (1M Na2CO3 [molecular weight {MW} =105.99], 20 mM HEPES buffer [MW =238.3] and sodium citrate). The negative control corresponded to distilled water (Daehan New Phram Co., Ltd, Gyeonggi, Korea.
ZnOSM20(−) NP preparation
The surface charge modification was performed by using sodium citrate to add topical negative charges to the ZnO NPs, as previously reported.19 The HEPES buffer solution was first adjusted to pH 7 using 1M Na2CO3, and then sodium citrate was added to the HEPES buffer to produce HEPES-citrate buffer (2% citrate). Next, the ZnO NPs were suspended in the HEPES-citrate buffer for chemical modification, as described previously,19 weighed, and resuspended in HEPES-citrate buffer solution to yield a high-dose (500 mg/mL) NP solution. The mid-dose (250 mg/mL) and low dose (125 mg/mL) NP solutions were diluted by suspending the modified ZnO NPs in sterile distilled water instead of HEPES-citrate buffer. Preparation of freshly modified ZnO NPs for use in each experimental group was done daily over the course of the 90-day study.
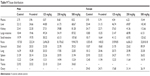
The stability and homogeneity of the resultant ZnOSM20(−) NPs were confirmed using method validation and verification of the formulation concentration according to protocols established by the Korea Testing and Research Institute (KTR), study number TBH-1026. The concentration of each preparation was measured on days 1, 45, and 90, just prior to administration to the rats. All preparations were confirmed within 100%±15% (Table 1).
 
|
Table 1 Results of dose formulation analysis in the 90-day oral toxicity study of ZnOSM20(−) NPs |
Test animals and experimental system
Five-week-old male and female, specific pathogen-free SD rats were purchased from Orient Bio, Inc. (Seongnam-si, Korea) and acclimated for 7 days before the initiation of the study. During the acclimation and experimental periods, the rats were housed in wire cages (maximum of two rats per cage) in a room with controlled temperature (22°C±3°C) and humidity (50%±20%) and a 12-hour light–dark cycle. The rats were fed a gamma ray-irradiated rodent diet (Cargill Agri Purina Korea Inc., Pyungtaek Seongnam Kyunggi-do, Korea) and filtered water ad libitum. The rats were divided into five groups (ten rats in each group, including five additional recovery animals in the negative control, vehicle control, and high-dose groups): 1) a negative control (distilled water) group; 2) a vehicle control (HEPES-citrate buffer) group; 3) a low-dose NP group; 4) a mid-dose NP group; and 5) a high-dose NP group. Three different kinds of studies were designed: 1) a primary 90-day oral toxicity study, followed by a 14-day recovery period; 2) a toxicokinetic study; and 3) an organ distribution study. Distilled water, HEPES-citrate buffer, and NP solutions were administered to the rats by gavage daily.
The dose levels in the 90-day oral toxicity study were determined based on the results of a previously conducted dose-finding, 14-day repeated oral toxicity study (KTR, study number TBH-1091). Significant adverse effects were observed at dose levels of 500 and 1,000 mg/kg in the dose-finding study; therefore, 500 mg/kg was selected for use in the high-dose group, and 250 and 125 mg/kg were selected for use in the mid- and low-dose groups, respectively, based on twofold intervals of NP dilution. In addition, three 14-day recovery groups were added to assess the persistence and reversibility of toxicity: 1) a negative control recovery group; 2) a vehicle control recovery group; and 3) a high-dose NP recovery group. The 14-day recovery period was initiated immediately after the end of the 90-day oral toxicity study.
The NP dosages in the toxicokinetic study and the organ distribution study were identical to those employed in the primary oral toxicity study. Details of the study design are summarized in Table 2.
The current study was conducted under the guidelines of the Organization for Economic Co-operation and Development of Good Laboratory Practices.
Animal observation
All animals in the primary oral toxicity study were observed once per day after the daily ZnOSM20(−) NP administration for general symptoms, the presence of any adverse/toxic symptoms, and/or the occurrence of death. In addition, all animals were observed once per week for chronic and tonic movements, repetitive behavior (excessive grooming, hyperactivity, or repetitive circling), abnormal behavior, aggression, motor coordination or lack thereof, gait, posture, and handling changes in response to NP administration.
Assessment of sensory reflexes and motor activity
The sensory response assessment for all animals in the primary oral toxicity study was determined at the end of the 90-day experimental period by assessing the grasp response, touch escape, vocalization, pupil reflex, blink reflex and response to toe pinch, tail pinch, and finger approach. Grip strength and motor activity were tested in the negative and vehicle control groups and the high-dose group at the end of the experimental period (n=5 animals per group). The grip strength in the front and hind limbs/feet was determined by using a rat grip strength measurement system (1027 CSX Grip Strength Meter; Columbus Instruments, Columbus, OH, USA). Grip strength was measured three times and averaged. A motor activity monitoring system (Columbus Instruments) and Truscan 99 data acquisition software (Deymed Diagnositics, Payette, ID, USA) were used to test motor activity.
Measurement of food intake, water consumption, and bodyweight
Food and water intake were measured once per week after the initiation of treatment, and the average daily intake (g/rat/day; n=5 animals per group) was calculated. Bodyweight was measured at animal acquisition, grouping, and once per week after the initiation of treatment.
Urinalysis
Urine samples were analyzed for nitrite, protein, glucose, ketone, urobilinogen, and bilirubin levels, specific gravity, pH, leukocyte count, and the presence of blood during the last week of the study by using a Clinitek 500 Urine Analyzer with a Multistix 10SG Urine Strip (Bayer, Morristown, NJ, USA) (n=5 animals per group). Fresh urine for the analysis was collected over a 3-hour period by using metabolic cages in the negative and vehicle control groups and the high-dose group (n=5 animals per group), followed by microscopic examination of the sediment. Urinary volume was measured by collecting the urine over a 24-hour period.
Ophthalmology examination
The visual appearance of the eye was inspected in the vehicle control, negative control, and high-dose groups before grouping and during the final week of the 90-day oral toxicity study (n=5 animals per group). In addition, fundoscopy was performed through the dilated pupil after dripping mydriatic fluid (Ocu-Tropine; Samil Pharmaceutical Co., Ltd., Seoul, Korea) into the eye with the aid of a fundus camera (Genesis, Kowa, Japan).
Hematology and serum biochemistry
All experimental and control animal groups were utilized for hematological analysis. Blood was withdrawn from the abdominal artery in animals that were fasted for 18 hours and then anesthetized with inhaled isoflurane. Three milliliters of blood was collected in a complete blood count collection tube (BD Biosciences, San Jose, CA, USA) containing EDTA (ethylenediaminetetraacetic acid) (EDTA 3K; BD Biosciences) for hematological analysis. Alternatively, 2.7 mL of blood was collected in a vacutainer tube (BD Biosciences) containing sodium citrate (sodium citrate 3.2%; BD Biosciences) for blood clotting. The rest of the blood was collected in a plain tube for serum biochemistry.
Hematological parameters were analyzed with an Advia 120E Automated Cell Counter (Siemens Healthcare, Malvern, PA, USA) for the following parameters: total leukocyte count, differential leukocyte count, total erythrocyte count, hemoglobin concentration, hematocrit, mean cell volume, mean cell hemoglobin (average mass of hemoglobin per red blood), mean cell hemoglobin concentration, total reticulocyte count, total eosinophil count, and total platelet count. Blood clotting time in the plasma was measured by centrifugation (3,000 rpm, 10 minutes), followed by analysis in an ACT 7000 Coagulation Analyzer (Werfen Medical, Lexington, MA, USA).
Serum biochemistry was measured after blood clotting at room temperature, separation of the serum by centrifugation (3,000 rpm, 10 min), and analysis in a Hitachi 7060 Automatic Serum Analyzer (Hitachi, Ltd, Tokyo, Japan). The following serological parameters were measured: total protein, albumin, albumin/globulin ratio, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatinine, blood urea nitrogen, total cholesterol, triglycerides, glucose, calcium, inorganic phosphorus, and creatine kinase. In addition, an electrolyte analyzer (EasyLyte PLUS Na/K/Cl Analyzer; Werfen Medical) was employed for sodium, potassium, and chloride analysis in the same serum sample.
Necroscopy, organ weights, and histopathology
Gross examination was performed of the external body surface of each rat, all orifices, the cranial cavity, all organs of the chest cavity, and the abdominal cavity. The liver, kidney, spleen, adrenal glands, testes, ovaries, brain, pituitary gland, lung, heart, thymus, uterus, prostate gland, epididymis, and submaxillary gland were removed, subjected to necroscopic analysis, and weighed to determine the wet weight of each organ. The relative organ-weight-to-bodyweight ratio was then calculated for each organ.
Next, the liver, kidney, adrenal gland, heart, lung, brain, pituitary gland, seminal vesicle, spleen, testes, ovaries, epididymis, prostate gland, uterus, vagina, tongue, trachea, esophagus, thymus, thyroid gland, stomach, duodenum, urinary bladder, small intestine, large intestine, eyeball, submandibular gland, pancreas, skin, mammary gland, sternum, femur, spinal cord, sciatic nerve, and mesenteric lymph node were removed and fixed in 10% neutral buffered formalin solution. The eyeball was fixed in Davidson solution, and the testes and epididymis were fixed in Bouin solution. Organs were stained with hematoxylin and eosin (H&E) or Alcian blue as appropriate and subjected to histopathological analysis. The analysis was only performed for the vehicle and negative control and high-dose groups, with the exception of the pancreas, stomach, and eyes, which were included in all groups.
Toxicokinetic study
For toxicokinetic analysis, blood samples were collected from the tail vein of all rats (negative control, vehicle control, and 125, 250, and 500 mg/kg groups) on the initial day of experimentation, and again on days 28 and 90 (n=3–9 animals per group). On day 1, blood samples were taken at 2 or 10 hours before the initiation of treatment in three animals; at 0.5, 4, or 24 hours after the initiation of treatment in another three animals; and at 1 or 6 hours after the initiation of treatment in the remaining animals. On day 28, blood was taken from 0.5 to 24 hours after the initiation of treatment in all animals; and on day 90, blood was taken at 1, 2, 4, 6, 10, or 24 hours after the initiation of treatment. At each time point, 1 mL aliquots of blood were placed into a heparinized microtube on ice. The plasma was then separated by centrifugation at 12,000 rpm and frozen at −80°C until use.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was performed after pretreatment of the serum samples with nitric acid and hydrogen peroxide, followed by heating at 160°C. Non-model interpretation based on hourly blood levels was used for the interpretation of toxicokinetic data. Maximum observed peak serum concentration (Cmax), the time at which Cmax was observed (Tmax), the area under the curve (AUC), and the half-life (T1/2) of elimination (up to 24 hours) were calculated by using Kinetica software (Thermo Electron Corp., Madison, WI, USA).20
Distribution of ZnOSM20(−) NPs in plasma, tissue, and feces
Blood samples (1 mL) from the tail vein and a defined amount of stool sample were collected. After removal of the fatty-tissue portions of the brain, liver, kidney, testes, ovaries, spleen, lung, stomach, small intestine, and large intestine, the organs were examined and weighed by using an ME254S analytical balance (Satorius, Goettingen, Germany). The distribution of the ZnOSM20(−) NPs was analyzed on the basis of Zn content in each organ by using an ULTIMA2 ICP-AAS spectrometer (HORIBA Jobin Yvon Horiba, Kyoto, Japan).
Statistical analysis
Levene’s test was performed to evaluate the homogeneity of variance. A one-way ANOVA (analysis of variance) for significance was performed to evaluate the bodyweight, food intake, water consumption, organ weight, and hematological and blood biochemical data. In cases where the data showed homogeneous variances and statistically significant differences between treatment and control groups, Scheffe’s post hoc test was conducted. In cases where the data showed heterogeneous variances and statistically significant differences between groups, Dunnett’s T3 post hoc test was conducted. All statistical analyses were performed by using freely available SPSS (IBM Corporation, Armonk, NY, USA) software, version 19.0.
Results
General observations
None of the animals died in any of the study groups during the course of the investigation. Excessive salivation was observed in the 125 mg/kg group from days 33 to 63, in the 250 mg/kg group from day 25 up until the end of treatment, and in the 500 mg/kg group from day 14 up until the end of treatment. Salivation was more frequent in the 500 mg/kg group relative to the other groups. Other adverse symptoms included piloerection, fur loss, soft stools, diarrhea, the appearance of wound on the skin and stain around the mouth, soiled fur, and opacity of the eye. However, no dose-response effects of the ZnOSM20(−) NPs were seen for any of these symptoms.
Sensor reflexes and motor activity
Abnormal behavior and functionality were not observed in any of the parameters for behavioral assessment or sensory reflex evaluation. Grip strength and motor activity were not treatment-related.
Effect of ZnOSM20(−) NPs on food intake, water consumption, and bodyweight
Bodyweight changes in the experimental and control groups were not significantly different for either sex (Figure 1), despite the fact that differences were observed in food and water intake, as noted below.
 
|
Figure 1 Bodyweight changes of male (A) and female (B) rats. |
For food intake, statistically significant increases (P<0.05) were observed in the 125 mg/kg group (both males and females) at weeks 6, 9, 12, and 13 compared with the control groups; in the 250 mg/kg group at weeks 5, 8, 9, 10, 11, 12, and 13 (P<0.05 or P<0.01); and in the 500 mg/kg group at weeks 3, 4, 5, 6, 8, 9, and 13 (P<0.05 or P<0.01), and during both weeks of the post-treatment recovery period (P<0.05 or P<0.01). When stratified by sex, food consumption significantly increased (P<0.05) in the female 250 mg/kg group at week 1 compared with the control groups, in the female 500 mg/kg group (P<0.05 or P<0.01) from weeks 1 to 13; and in the female 500 mg/kg recovery group at week 2.
In male rats, water consumption significantly increased in all groups at various time points. This was most obvious in the 500 mg/kg group compared with the controls and occurred at weeks 1, 2, 3, 4, and 12, and during week 2 of the recovery period (P<0.05 or P<0.01). Significantly increased water consumption was also seen in the 250 mg/kg group at weeks 3 and 4 (P<0.05), and in the 125 mg/kg group at week 4 (P<0.05). In females, water consumption was significantly increased (P<0.05 or P<0.01) in the 500 mg/kg group at weeks 1, 2, 3, 4, and 5, and in 500 mg/kg recovery group at week 2.
Urine and ophthalmology testing
Abnormal urinary changes were not observed in any of the treated animals compared with the controls. One case of opacity of the eye in the male 500 mg/kg group was observed.
Necropsy and organ weight
The necropsy results revealed one case of a mass in the thymus in the male 250 mg/kg group, and one case of a small testis with seminal vesicle atrophy in the male 500 mg/kg group. In the females, one case of black spot was observed on the right adrenal gland in the control group, in addition to one case of a brownish change on the left lateral lobe of the liver in the 500 mg/kg group. Necropsy findings showed no dose dependency of any of these findings.
The absolute and relative organ weights in the 90-day oral toxicity study were statistically similar between the experimental groups and the control groups – shown for the absolute adrenal gland weight in the males (Table 3), and the absolute ovary and uterus weights in the females (Table 4). However, the absolute organ weight of the submandibular gland was significantly increased (P<0.05) in the male 500 mg/kg recovery group compared with the controls (Table 4). The relative organ weight of the adrenal relative weight was also significantly increased (P<0.05) in the male 500 mg/kg group, and the relative uterus weight was significantly increased (P<0.05) in the female 500 mg/kg recovery group (Tables 3 and 4). Nonetheless, these organ weight differences showed no dose dependency or correlation with the histopathologic findings described in the Histopathological examination section.
Effects of ZnOSM20(−) NPs on clinical chemistry and hematology
Hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were all significantly decreased (P<0.05) in the male 250 and/or 500 mg/kg groups compared with the controls (Table 5). Furthermore, eosinophil counts were significantly increased (P<0.05) in the male 250 mg/kg group (Table 5), and erythrocyte counts were significantly increased (P<0.05) in the male 500 mg/kg recovery group (Table 6). Likewise, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were significantly decreased (P<0.05 or P<0.01) in the female 500 mg/kg group (Table 5). In addition, total erythrocyte counts were significantly increased (P<0.05 or P<0.01) in the female 500 mg/kg group and the female 500 mg/kg recovery group (Tables 5 and 6).
Total serum protein and albumin levels were significantly decreased (P<0.05) in the male 250 and/or 500 mg/kg groups compared with the control groups, and creatine kinase levels were significantly increased (P<0.05) in the male 500 mg/kg group (Table 7). Similarly, total serum protein and albumin levels were significantly decreased (P<0.05) in the female 250 and 500 mg/kg groups compared with the controls (Table 7). None of the other investigated hematological parameters (leukocyte, platelet, or reticulocyte counts, albumin/globulin ratio, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatinine, blood urea nitrogen, total cholesterol, triglycerides, glucose, calcium, inorganic phosphorus, sodium, potassium, and chloride) differed significantly between the groups.
Histopathological examination
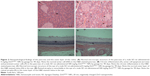
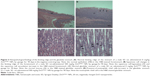
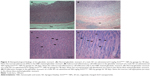
Most of the organs appeared normal in the experimental groups. However, acinar cell apoptosis, ductular hyperplasia, periductular lymphoid cell infiltration, and regenerative acinar cells were all observed in the pancreas of the male and female 500 mg/kg groups, and minimal-to-severe grade retinal atrophy was observed in the male 250 and 500 mg/kg groups and the female 500 mg/kg group (illustrated for a male rat in the 500 mg/kg group in Figure 2). In all treatment groups, various kinds of gastric lesions in varying grades were observed. Therefore, we examined these lesions in more detail by dividing the stomach into three parts: the forestomach, the limiting ridge, and the glandular stomach (Figures 3 and 4).
Epithelial vacuolation was observed in the forestomach, and basal cell hyperplasia, epithelial hyperplasia, epithelial vacuolation, hyperkeratosis, and submucosal inflammatory cell infiltration were observed in the limiting ridge (Figures 3 and 4). In the glandular stomach, erosive lesions, infiltrating epithelial globule leukocytes, submucosal edema, and inflammation, chief cell-like cells with eosinophilic cytoplasmic granules, and a reduced number of parietal cells were all observed (grade: minimal-to-severe, Figures 3 and 4). The grade and incidence of these lesions was reduced in the recovery group (data not shown).
Toxicokinetic study
On the first day of ZnOSM20(−) NP administration, Cmax values were fairly similar in the male and the female 125, 250, and 500 mg/kg groups (47.38–67.03 μg/mL) (Table 8) and were not dose-dependent. However, they increased in a dose-dependent manner in both sexes on days 28 and 90.20
In general, the AUC values were dose-dependent in males and females on all three days, with the exception of the first day of administration in males (Table 8).
Tmax was 4–6 hours, regardless of the day of NP administration, and the average T1/2 in the blood was 1–13 hours (Table 8).
Distribution of ZnOSM20(−) NPs in the plasma, organs, and feces
The distribution of ZnOSM20(−) NPs in the plasma, stools, brain, liver, kidney, testis, ovary, spleen, lung, stomach, small intestine, and large intestine was analyzed by using a demonstrated ICP-OES (inductively coupled plasma optical emission spectrometry) method. No clear differences were observed between the data for the male and female rats (Table 9). However, Zn concentrations dose-dependently increased in the liver, kidney, intestine, and plasma of the experimental compared with the control groups. The ZnOSM20(−) NPs were also dose-dependently excreted into the feces, as evidenced by high Zn levels. On the other hand, little or no increase was found in the Zn concentration in the brain, testis, ovary, spleen, stomach, or lung, with the exception of the stomach in the female 500 mg/kg group (Table 9). Thus, the histopathological findings regarding the ZnOSM20(−) NP-induced lesions in the eye, pancreas, and stomach did not show good correlation with the distribution data.
|
Table 9 Tissue distribution |
Discussion
The current manuscript describes a 90-day repeated dose, subchronic oral toxicity study designed to investigate the NOAEL and systemic toxicity of ZnOSM20(−) NPs in SD rats. During the test period, none of the animals died. Nevertheless, a number of adverse symptoms were associated with the NPs, including salivation in all of the test animals. Opacity of the eye was also observed, albeit in only one rat in the male 500 mg/kg group, and was considered to be related to the more general pathologic findings of retinal atrophy. Evaluation of sensory responses, motor activity, weight changes, and urinalysis were unremarkable in the experimental groups of both sexes compared with the control groups. At some time points, and particularly during the early stages of ZnOSM20(−) NP administration, food intake, and water consumption were increased in the experimental versus the control groups, but this phenomenon was not thought to be treatment-related due to the lack of dose dependency or correlation with bodyweight changes.
Hematological and blood biochemical analyses revealed small but significant decreases in the amount of hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration in the male 250 and/or 500 mg/kg groups and in the female 500 mg/kg group. Moreover, total serum protein and albumin levels were significantly decreased in the 250 and/or 500 mg/kg groups for both males and females. These notable reductions in hematologic parameters were regarded as NP-related changes and were likely due to the fact that excessive amounts of Zn ions induce iron deficiency anemia, as well as sideroblastic anemia and bone marrow depression.21,22
In the current study, apoptosis of pancreatic acinar cells and infiltration of periductular lymphoid cells were observed as dose-dependent changes in both male and female experimental groups, along with minimal to marked ductular epithelial hyperplasia and increased numbers of regenerated acinar cells. A previous 90-day toxicity study of ZnO NPs reported that the manufactured NPs induced lesions attributable to pancreatitis.23 Similarly, another study showed that the 14-day oral administration of 20 nm ZnO NPs in mice induced mild infiltration of inflammatory cells in the liver and pancreas, with severe stomach mucosal damage.24 In addition, the oral administration of 20 nm ZnO NPs and ZnO microparticles in SD rats provoked histological lesions in the liver, pancreas, heart, and stomach. The incidence of the lesions was higher with low-dose 20 nm NPs than with high-dose microparticles.25 Although the lesions tended to resolve within 2 weeks of recovery, they were severe enough to induce functional abnormalities, and were therefore thought to be of toxicological significance. Considering the results of the above investigations and our current results, the pancreas and stomach were apparently affected by orally administered ZnOSM20(−) NPs due to continuous irritation.
In the present report, retinal atrophy of the eye was observed in the 250 and 500 mg/kg male groups and the 500 mg/kg female group, but not in the corresponding control groups. Therefore, this condition was consistent with a treatment-related injury, because zinc induces retinal damage when it is administrated at high concentrations. By contrast, zinc can prevent retinal disease (ischemia) when administrated at low concentrations.26
Various histopathological lesions were also observed in the stomach of the ZnOSM20(−) NP-treated rats. For example, lesions were found along the limiting ridge that were characteristic of epithelial cell hyperplasia. In addition, submucosal inflammation and chief cell-like cells with cytoplasmic eosinophilic granules were evidenced in the glandular stomach in the experimental animals, but not in the control group. In the recovery group, the pancreas and stomach lesions resolved, but the retinal atrophy did not (data not shown). These results indicate that the target organs of the ZnOSM20(−) NPs are the pancreas, stomach, and eye.
A recent report showed that ZnO NPs can be toxic to human cancer cells and mitochondria.27 Another report demonstrated that ZnO NPs exhibit selective cytotoxic actions against rapidly proliferating cells.28 Along the same lines, our histopathological data showed that the predominant lesions in ZnOSM20(−) NP-treated rats involved actively proliferating cells, such as epithelial cells and regenerative acinar cells.
Toxicokinetic data showed similar dose- and time-dependent increases in the accumulation and absorption of Zn stemming from ZnOSM20(−) NPs in the liver, kidney, large intestine, and small intestine of both male and female rats. By contrast, absorption and accumulation of ZnO in the brain, testes, ovaries, spleen, stomach, and lung were not significantly different in either male or female rats compared with the controls. Thus, the ZnOSM20(−) NPs exhibited no sex specificity. Furthermore, the primary pathologic lesions in the pancreas, stomach, and eye following ZnOSM20(−) NP administration did not correlate with the distribution data.
Conclusion
The present 90-day repeated dose, subchronic oral toxicity investigation of ZnOSM20(−) NPs in SD rats explored the actions of the NPs at three dose levels: 125, 250, and 500 mg/kg of bodyweight. The results failed to demonstrate an NOAEL for the NPs, but the LOAEL in this study was 125 mg/kg. Systemic adverse effects included increased salivation, acinar cell apoptosis, chronic inflammation in the pancreas, inflammatory stomach lesions, retinal atrophy of the eye, and changes in anemia-related parameters. These observations indicate that ZnOSM20(−) NPs must be used with caution in human medicine.
Acknowledgments
This study was supported by a grant (10182MFDS991) from the Korean Ministry of Food and Drug Safety in 2010 and by the Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Korea.
Disclosure
The author reports no conflicts of interest associated with this work.
References
Oberdõrster G, Oberdõrster E, Oberdõrster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. | ||
Warheit DB, Borm PJA, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19(8):631–643. | ||
Pillai SC, Kelly JM, Ramesh R, McCormack DE. Advances in the synthesis of ZnO nanomaterials for varistor devices. J Mater Chem C. 2013;1(20):3268–3281. | ||
Rauwel E, Galeckas A, Rauwel P, Sunding MF, Fjellvag H. Precursor-dependent blue-green photoluminescence emission of ZnO nanoparticles. J Phys Chem C. 2011;115(51):25227–25233. | ||
Jiang J, Oberdõrster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11(1):77–89. | ||
Oberdorster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2(1):8. | ||
Ball P. Natural strategies for the molecular engineer. Nanotechnology. 2002;13:R15. | ||
Roco MC. Broader societal issues of nanotechnology. J Nanopart Res. 2003;5(3):181–189. | ||
Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. | ||
Brunner TJ, Wick P, Manser P, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40(14):4374–4381. | ||
Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37(3):251–277. | ||
Klaine SJ, Alvarez PJ, Batley GE, et al. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27(9):1825–1851. | ||
Kreyling WG, Semmler M, Erbe F, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65(20):1513–1530. | ||
Yamago S, Tokuyama H, Nakamura E, et al. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem Biol. 1995;2(6):385–389. | ||
Jani P, Halbert G, Langridge J, Florence A. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J Pharm Pharmacol. 1989;41(12):809–812. | ||
Park D-H, Li L, Jang H-K, et al. Biological safety and anti-hepatofibrogenic effects of Brassica rapa (turnip) nanoparticle. Mol Cell Toxicol. 2009;5(4):317–322. | ||
Buzea C, Pacheco I, Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2(4):MR17–MR71. | ||
Hong T-K, Tripathy N, Son H-J, Ha K-T, Jeong H-S, Hahn Y-B. A comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J Mater Chem B. 2013;1(23):2985–2992. | ||
Kim K-M, Kim T-H, Kim H-M, et al. Colloidal behaviors of ZnO nanoparticles in various aqueous media. Toxicol Environ Health Sci. 2012;4(2):121–131. | ||
Baek M, Chung HE, Yu J, et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomedicine. 2012;7:3081–3097. | ||
Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–1365. | ||
Broun ER, Greist A, Tricot G, Hoffman R. Excessive zinc ingestion. JAMA. 1990;264(11):1441–1443. | ||
Seok SH, Cho WS, Park JS, et al. Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: biopersistence of nanoparticles and possible solutions. J Appl Toxicol. 2013;33(10):1089–1096. | ||
Wang B, Feng W, Wang M, et al. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res. 2007;10(2):263–276. | ||
Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya BM. Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health. 2012;28(8):675–686. | ||
Ugarte M, Osborne NN. Zinc in the retina. Prog Neurobiol. 2001;64(3):219–249. | ||
Li J-h, Liu X-r, Zhang Y, et al. Toxicity of nano zinc oxide to mitochondria. Toxicol Res. 2012;1(2):137. | ||
Taccola L, Raffa V, Riggio C, et al. Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine. 2011;6:1129–1140. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.