Back to Journals » Clinical Interventions in Aging » Volume 9
24-weeks Pilates-aerobic and educative training to improve body fat mass in elderly Serbian women
Authors Ruiz-Montero PJ, Castillo-Rodriguez A, Mikalački M, Nebojsa, Korovljev D
Received 26 July 2013
Accepted for publication 24 September 2013
Published 31 January 2014 Volume 2014:9 Pages 243—248
DOI https://doi.org/10.2147/CIA.S52077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Pedro Jesús Ruiz-Montero,1 Alfonso Castillo-Rodriguez,2 Milena Mikalacki,3 Čokorilo Nebojsa,3 Darinka Korovljev3
1Department of Physical Education and Sport, Faculty of Physical Activity and Sport, University of Granada, Granada, Spain; 2Faculty of Sport, University of Pablo de Olavide, Seville, Spain; 3Faculty of Sport and Physical Education, University of Novi Sad, Novi Sad, Serbia
Background: The purpose of this study was to examine the differences in anthropometric measurements using an aerobic and Pilates exercise program which lasted 24 weeks.
Method: This was a clinical intervention study of 303 women over the age of 60 living in Novi Sad, Serbia. Changes in body mass index and skinfold thickness were estimated through height, weight, and anthropometric measurements. The program comprised Pilates exercises for upper- and lower-body strength, agility, and aerobic capacity.
Results: Fat mass (FM) improved significantly (pre-test, 32.89%, 8.65; post-test, 28.25%, 6.58; P<0.01). Bone diameters and muscle perimeters showed no significant changes pre- and post-test (P>0.05), but there was a higher correlation between FM (%) and waist–hip ratio (rho, 0.80; P<0.01).
Conclusion: A mixed program of aerobics and Pilates, controls and improves baseline muscle mass and decreases FM values, without causing deterioration during practice and follow-up exercises.
Keywords: lean body mass, anthropometric measures, educative program
A Letter to the Editor has been received and published for this article.
Introduction
Changes in body composition can very often cause chronic diseases, and as a result have become a primary concern.1 Lean body mass (LBM) and fat mass (FM) are body composition states that change continuously and are closely related to the aging process when compared with other factors.2,3 Moreover, a strong link has been established between decreased muscle strength, physical capacity, quality of life, and the loss of fat-free mass.4
These changes can be seen in variations in body composition after middle age,5 through the increase of FM, reduction in LBM,6,7 and through the loss of height due to compression of the vertebrae and progressive curvature of the back.8 Consequently, another deterioration has been identified from the age of approximately 65 to 75–80,9 where the loss of muscle mass becomes 25%.10
Physical activity (PA) is considered to be one of the most important health indicators which produces benefits for all age groups,11–13 is used as an effective intervention to prevent functional loss related to age,14–17 and promotes an improved quality of life and, as a result, greater longevity, coupled with appropriate eating patterns.18–21 Physical exercise also improves quality of life due to the reduction of FM and obesity,22,23 and prevents a rapid reduction in the size and number of muscle fibers.24
The practice of exercise should be consistent and controlled by specific programs. If the subject chose to do the opposite, ie, not follow a specific exercise program, body composition might not be affected.25 Thanks to a progressive strength program which lasted 12 months, improvements in the health of elderly women with metabolic diseases were reflected in their body composition.26 According to Sims et al there are studies that do not show clear links between PA programs and changes in FM in women aged between 50 and 79, and which do not show prevention of loss of LBM in aerobic activities, regardless of the level.27
However, PA in the elderly is a widely studied and corroborated factor that improves quality of life.28 In turn, it helps to reduce the loss of functionality and the implications of such during the aging process.29 The American College of Sports Medicine (ACSM)30 recommends, as part of a guide on basic exercises, that the elderly use training programs that focus on resistance, strength, aerobic capacity, and flexibility exercises. Part of its content focuses on the Pilates method through the activation of brain cells, stimulation of the mind, and the positive impact on the body.31 The ACSM ranked this method in seventh place as an emerging PA in 2008 and 2009,32 and in the US alone, the number of users reached 10.5 million in 2004. The Pilates method coincides with the modern principles of fitness, personal training, and mental happiness through exercises that maintain a neutral spine position and appropriate use of the floor and equipment to develop strength and balance.33
Aerobic capacity and endurance can also help to maintain muscle mass and strength in the elderly as well as the Pilates method.22 According to De Cocker et al, there are relatively few studies related to muscle strengthening activities compared with other aerobic activities.34 Some of the studies provide beneficial effects, while others do not offer any significant changes35 in terms of intensity, duration, frequency,36 or activity.35
This research project aimed to assess the differences in body composition and anthropometric measurements of a sample of Serbian women over the age of 60, in a 24-week clinical intervention study, through a guided program that combined aerobics and Pilates.
Methods
Participants
A total of 303 elderly Serbian females and eight males over the age of 60 voluntarily participated in this study. It was decided to exclude the men due to their small number. Subjects were informed about the experimental procedures, completed a medical history form, and signed an informed consent statement. The inclusion criteria were that the participants were women aged greater than 60 and less than 70 years. The exclusion criteria included having any chronic disease, limited functional mobility and taking medications. Thirty participants were smokers. A blood test was carried out to check that the participants had no problems related to the following biological parameters: glucose (4.68 g/L), triglycerides (1.29 mmol/L), high density lipoprotein (1.48 mmol/L), and low density lipoprotein (3.78 mmol/L). The average age for the onset of menopause was 48.86 years. The average number of pregnancies was 1.92. No participants were medicated during the training process, and none of the participants performed any other PA.
Measurements obtained were: height, weight, body mass index, subcutaneous skinfold thickness (triceps, subscapularis, suprailiac, abdomen, front thigh, and medial calf), muscular girths (upper arm relaxed, upper arm flexed and tensed, abdominal, thigh, and maximum calf), and four diameters (wrist, femur, humeral, and ankle).
Instruments
Bodyweight was measured to ±0.1 kg on an electronic scale (Seca GmbH & Co, KG, Hamburg, Germany), with participants wearing light indoor clothing and no shoes. Measurements were performed following the standardized techniques adopted by the International Society for the Advancement of Kinanthropometry.37 The technical error of measurement was lower than 5% for skinfold thickness and lower than 1% for the other measurements. Skinfold thickness was taken using a caliper with a precision of 0.2 mm (Holtain Ltd, Crymych, UK). Girths were performed with a flexible metallic tape measure with a precision of 0.1 mm. Body mass index was calculated as weight (kg) × height2 (m). The equation used to determine FM was: corporal density of Durnin–Womersley38 =1.1567 − 0.0717 × log (subscapularis skinfold + triceps skinfold + forearm skinfold + biceps skinfold) and FM equation39 (%) = [4.57/corporal density − 4.142] × 100.
Procedure
This present study was initiated in order to establish the influence of PA (through a program of educational activity and PA based on Pilates and aerobics) on the elderly, with different bodyweights and FM. A medical and sociodemographic questionnaire was completed to collect social, demographic, and medical variables. Consequently, the collection of anthropometric measurements was carried out.
Data were collected twice. On the first occasion, a pre-test was evaluated during the month of May 2011. Then, a post-test was performed in November of 2011. The Pilates-gym training process lasted for 24 weeks. The program had an 80% attendance rate during this period.
The training program consisted of music-based aerobics and Pilates, basic to intermediate level. Following these sessions, concepts of health education were given to orientate participants towards a more healthy posture and the practice of food hygiene in their daily lives. The diet regime was established according to the recommendations of ACSM13 and was followed by 91% of the participants. The sessions included in this program took place twice per week and lasted 55–60 minutes per day-session, with an effective PA time of 45 minutes, in line with the requirements laid out by the ACSM.23 The program comprised Pilates exercises for upper- and lower-body strength, agility, and aerobic capacity. During the first session of the intervention process, participants were asked their perceived level of exertion on a scale of 0–10 (Borg scale) to establish the initial level and then gradually increase exercise intensity. This course lasted 24 weeks and was based initially on a pilot study in which changes in behavior and body composition of the participants were expected.40 This study was approved by the Ethical Committee of the University of Novi Sad.
Statistical analyses
Data were analyzed using the SPSS (SPSS, Chicago, IL, USA) statistical program, version 17.0. Normality of distribution was tested by means of the Kolmogorov–Smirnov test, which suggested that the variables were not distributed normally. Descriptive statistical methods were used for the calculations of the means and standard deviations. Repeated-measurement tests were used to determine the differences between times (Wilcoxon test; repeated-measures test). For all analyses, significance was accepted at P<0.05.
Results
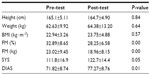
Characteristics of the subjects are shown in Table 1. Participants had a lower height and had gained more weight after the training period, although these data were not significant. However, the FM was lower after the Pilates-gym program (P<0.01). Blood pressure, both systolic and diastolic, increased (P<0.05).
  | Table 1 Descriptive characteristic of participants |
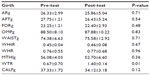
Muscle perimeters were all lower, apart from in the waist. These data show no significant differences between pre- and post-test (Table 2). Waist-to-thigh ratio was higher post-test than pre-test.
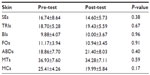
As for skinfold thickness, various results were obtained (P<0.05). In some participants, they were higher such as skinfold thickness of triceps, inner forearm, and the abdomen, and in others, the results were lower, such as in skinfold thickness in the subscapularis, outer forearm, mid-thigh, and calf (Table 3).
Lastly, bone diameters were similar in the pre- and post-tests (P>0.05) (Table 4). Regarding correlations, there was a strong relationship between FM (%) and waist-to-hip ratio (rho, 0.80; P<0.01).
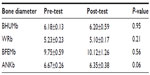  | Table 4 Wilcoxon test of bone diameters |
Discussion
This is the first study assessing the effects of 24 weeks of Pilates-aerobic exercise on older, Serbian women. The aim of this study was to examine body composition (FM and LBM) at the beginning and end of an aerobic-Pilates program. To ensure a full collaboration of participants over the period of 24 weeks, it was especially important that they were motivated in these longitudinal studies so that the practice of PA was done on a daily basis.41
When there is an increase in FM, there is a reduction in muscle strength, physical capacity, and quality of life, along with a loss of fat-free mass.4 The elderly, with more sedentary lifestyles, lose fat-free mass faster than people who perform PAs through a combination of strength training42 and force balance training,43 the latter being the reason for the prevention of weight loss and in maintaining functional capacity in people above the age of 70.44 Low mortality values and less muscle fat have even been linked to an increase in aerobic exercise through walking speed.44 This body fat (%) is related to the waist-to-hip ratio, as in the study by Bae et al1 with a sample of the elderly. However, the correlation between the two that we have obtained is stronger (r=0.80; P<0.01) due to the homogeneity of the study sample.
Joseph Pilates developed a system of exercises which is defined as “a unique method of physical fitness that uses a combination of muscle strengthening, lengthening and breathing to develop trunk muscles and restore muscle balance.”45 The Pilates method is considered by European practice guidelines as an adequate treatment for correct modification of posture,46,47 but it is not effective in the diminution of disability.48 Despite there being no clear evidence of improved functionality in elderly people through the practice of Pilates, except balance,49,50 an improvement in body composition in healthy people has been found,51 as observed in the present study, as well as a reduction in risk situations and cardiovascular mortality.52 Various aerobic exercise programs have shown improvements in functional capacity and particularly cardiovascular type exercises in women during the aging process.53 The participants had an improved FM level of 32.89%±8.65% and at the end of the Pilates-aerobic program (24 weeks), the FM levels had decreased to 28.25%±6.58%. Skinfold thickness variations were very small (P>0.05); however, Table 3 shows that in all cases it decreases. Therefore, the sum of these skinfolds is a major difference. This is the reason why FM is highly significant (P<0.01). This decline in FM is linked to the completion of PA controlled and planned,41 in addition to following a balanced diet. However, other authors such as Dias et al54 and Lim et al55 have not found any improvements in their participants who took part in a Pilates program. The reason for this is thought to be due to the short duration of the course (6 weeks).
FM in the elderly does not only remain stable through the practice of the Pilates-aerobic program, but it can also decrease significantly (Table 1). Hence, as a result of the human aging process, there is a considerable loss of skeletal muscle mass over time, especially in women.53 However, our results are similar pre-post after 24 weeks of PA (P>0.05), although Pereira et al report an increase in skeletal muscle mass, thanks to the Pilates programs.
Conclusion
In conclusion, a combined program of aerobic and Pilates, carried out under the supervision of an instructor, at least twice a week, produces health benefits in functionally independent women over the age of 60. Furthermore, initial muscular mass values remained stable, whereas FM decreased, without causing deterioration of health during the practice of exercises. For the level of balance and functional performance, physical capacity was not evaluated. We need to conduct further research, using fitness tests and comparing benefits of physical programs, especially Pilates and aerobic activity. Finally, it is important to note that the present study has its limitations with this population. According to some authors, data of the Serbian elderly sample is limited56 because there are not many studies related to this area of Europe and in particular regarding the elderly.
Acknowledgments
A special thanks to all the women who participated in this study, and to any inconvenience it may have caused them. The authors also thank the final-year students at the Faculty of Sport and Physical Education, University of Novi Sad (Serbia), who collaborated with the project and organized some of the activities and content of the Pilates and aerobics sessions, as well as helped with the assessments. Thanks also to the physical intervention group al-Andalus and research group CTS-545 (Department of Physical Education and Sport, University of Granada) for their various forms of collaboration.
Funding
This study was funded by the Provincial Secretariat for Science and Technological Development in Novi Sad, and was entitled “The Influence of physical activity on risk factors in the working population” (number:114-451-2337/2011-01).
Disclosure
The authors report no potential conflicts of interest in this research and/or publication of this article.
References
Bae CY, Kang YG, Suh YS, Han JH, Kim SS, Shim KW. A model for estimating body shape biological age based on clinical parameters associated with body composition. Clin Interv Aging. 2013;8:11–18. | |
Poehlman ET, Toth MJ, Fishman PS, et al. Sarcopenia in aging humans: the impact of menopause and disease. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:73–77. | |
He Q, Heo M, Heshka S, et al. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr. 2003;78(1):72–77. | |
Katula JA, Sipe M, Rejeski WJ, Focht BC. Strength training in older adults: an empowering intervention. Med Sci Sports Exerc. 2006;38(1):106–111. | |
Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–239. | |
Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1485–R1495. | |
Colado JC, Garcia-Masso X, Rogers ME, Tella V, Benavent J, Dantas EH. Effects of aquatic and dry land resistance training devices on body composition and physical capacity in postmenopausal women. J Hum Kinet. 2012;32:185–195. | |
Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the Baltimore Longitudinal Study of Aging. Am J Epidemiol. 1999;150(9):969–977. | |
Kyle UG, Genton L, Hans D, et al. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc. 2001;49(12):1633–1640. | |
Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–E101. | |
Crombie IK, Irvine L, Williams B, et al. Why older people do not participate in leisure time physical activity: a survey of activity levels, beliefs and deterrents. Age Ageing. 2004;33(3):287–292. | |
Bergamin M, Zanuso S, Alvar BA, Ermolao A, Zaccaria M. Is water-based exercise training sufficient to improve physical fitness in the elderly? A systematic review of the evidence. Eur Rev Aging Phys Act. 2012;9:129–141. | |
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. | |
Henwood TR, Taaffe DR. Short-term resistance training and the older adult: the effect of varied programmes for the enhancement of muscle strength and functional performance. Clin Physiol Funct Imaging. 2006;26(5):305–313. | |
Ikezoe T, Mori N, Nakamura M, Ichihashi N. Age-related muscle atrophy in the lower extremities and daily physical activity in elderly women. Arch Gerontol Geriatr. 2011;53:e153–e157. | |
Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. | |
Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. | |
Newby PK, Weismayer C, Akesson A, Tucker KL, Wolk A. Long-term stability of food patterns identified by use of factor analysis among Swedish women. J Nutr. 2006;136(3):626–633. | |
Guedes DP, Hatmann AC, Martini FA, Borges MB, Bernardelli R Jr. Quality of life and physical activity in a sample of Brazilian older adults. J Aging Health. 2012;24(2):212–226. | |
Vuori IM. Health benefits of physical activity with special reference to interaction with diet. Public Health Nutr. 2011;4:517–528. | |
Wärnberg J, Gomez-Martinez S, Romeo J, Díaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–175. | |
Hayes LD, Grace FM, Sculthorpe N, et al. The effects of a formal exercise training programme on salivary hormone concentrations and body composition in previously sedentary aging men. Springerplus. 2013;2(1):18. | |
Serra JR. Prescripción de Ejercicio Físico Para la Salud [Prescription of physical exercise for health]. Barcelona: Paidotribo; 1996. Spanish. | |
Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–M724. | |
Maggioni MA, Ce E, Giordano G, et al. Effects on body composition of different short-term rehabilitation programs in long-stay hospitalized elderly women. Aging Clin Exp Res. 2012;24(6):619–626. | |
Mavros Y, Kay S, Anderberg KA, et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care. 2013;36(8):2372–2379. | |
Sims ST, Kubo J, Desai M, et al. Changes in physical activity and body composition in postmenopausal women over time. Med Sci Sports Exerc. 2013;45(8):1486–1492. | |
Colado JC, Triplett NT. Effects of a short-term resistance program using elastic bands versus weight machines for sedentary middle-aged women. J Strength Cond Res. 2008;22(5):1441–1448. | |
Castillo MJ, Ruiz JR, Ortega FB, Gutiérrez A. Anti-aging therapy through fitness enhancement. Clin Interv Aging. 2006;1(3):213–220. | |
American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. | |
Pilates JH, Miller WJ. Pilates’ Return to Life through Contrology. New York: JJ Augustin; 1945. | |
Thompson WR. Worldwide survey reveals fitness trends for 2009. ACSM´s Health Fitness J. 2009;12(6):7–14. | |
Levine B, Kaplanek B, Jaffe WL. Pilates training for use in rehabilitation after total hip and knee arthroplasty: a preliminary report. Clin Orthop Relat Res. 2009;467(6):1468–1475. | |
De Cocker KA, De Bourdeaudhuij IM, Brown WJ, Cardon GM. Effects of “10,000 steps Ghent”: a whole-community intervention. Am J Prev Med. 2007;33(6):455–463. | |
Braz NF, Carneiro MV, Oliveira-Ferreira F, et al. Influence of aerobic training on cardiovascular and metabolic parameters in elderly hypertensive women. Int J Prev Med. 2012;3(9):652–659. | |
Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–725. | |
Ross WD, Marfell-Jones MJ. Kinanthropometry. In: Mac Dougall JD, Wenger HA, Green HJ, editors. Physiological Testing of the High Performance Athlete. Champaign, IL: Human Kinetics Books; 1991:223–308. | |
Durnin JV, Womersley J. Body fat assessed from-total body density and its estimation from skinfolds thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. | |
Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci. 1963;110:113–140. | |
Burke L, Howat P, Lee AH, Jancey J, Kerr D, Shilton T. Development of a nutrition and physical activity booklet to engage seniors. BMC Res Notes. 2008;1:77. | |
Bocalini DS, Lima LS, de Andrade S, et al. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin Interv Aging. 2012;7:551–556. | |
Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–650. | |
Evans WJ. Exercise and nutritional needs of elderly people: effects on muscle and bone. Gerodontology. 1998;15(1):15–24. | |
Woo J, Yu R, Yau F. Fitness, fatness and survival in elderly populations. Age (Dordr). 2013;35(3):973–984. | |
Cozen DM. Use of Pilates in foot and ankle rehabilitation. Sports Med Arthrosc. 2000;8(4):395–403. | |
Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. 3rd ed. Baltimore, MD: Williams and Wilkins; 2007. | |
Delitto A, George SZ, Van Dillen LR, et al. Low back pain. J Orthop Sports Phys Ther. 2012;42:A1–A57. | |
Pereira LM, Obara K, Dias JM, et al. Comparing the Pilates method with no exercise or lumbar stabilization for pain and functionality in patients with chronic low back pain: systematic review and meta-analysis. Clin Rehabil. 2012;26:10–20. | |
Kaesler DS, Mellifont RB, Kelly PS, Taaffe DR. A novel balance exercise program for postural stability in older adults: a pilot study. J Bodyw Mov Ther. 2007;11:37–43. | |
Babayigit Irez B, Ozdemir RA, Evin R, Irez SG, Korkusuz F. Integrating Pilates exercise into an exercise program for 65+ year-old women to reduce falls. J Sports Sci Med. 2011;10:105–111. | |
Fett CA, Fett WCR, Oyama SR, Marchini JS. Body composition and somatotype in overweight and obese women pre- and post-circuit training or jogging. Rev Bras Med Esporte. 2006;12(1):45–50. | |
Lavie CJ, Milani RV, Marks P, de Gruiter H. Exercise and the heart: risks, benefits, and recommendations for providing exercise prescriptions. Ochsner J. 2001;3(4):207–213. | |
Lavie CJ. Making exercise and fitness a high priority. Ochsner J. 2007;7(4):154–157. | |
Dias R, Prestes J, Manzatto R, et al. Efeitos de diferentes programas de exercício nos quadros clínico e funcional de mulheres com excesso de peso [Effects of different exercise programs in clinic and functional status of overweight women]. Rev Bras Cineantropom Desempenho Hum. 2006;8(3):58–65. Portuguese. | |
Lim EC, Poh RL, Low AY, Wong WP. Effects of Pilates-based exercises on pain and disability in individuals with persistent nonspecific low back pain: a systematic review with meta-analysis. J Orthop Sports Phys Ther. Feb 2011;41(2):70–80. | |
Popovic S, Bjelica D, Molnar S, Jaksic D, Akpinar S. Body height and its estimation utilizing arm span measurements in Serbian adults. Int J Morphol. 2013;31(1):271–279. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.